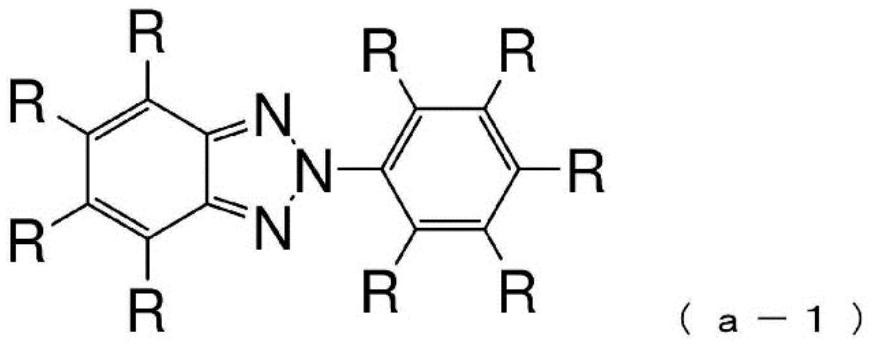
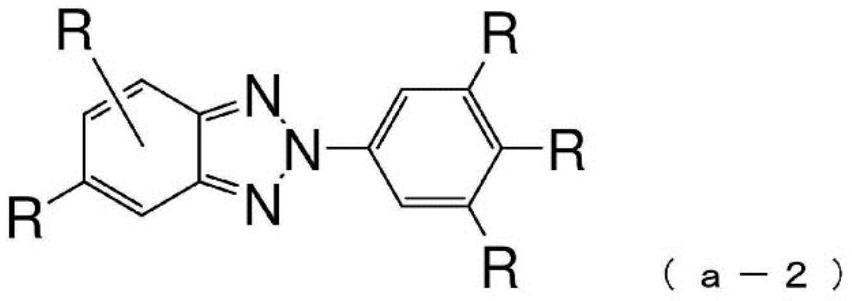
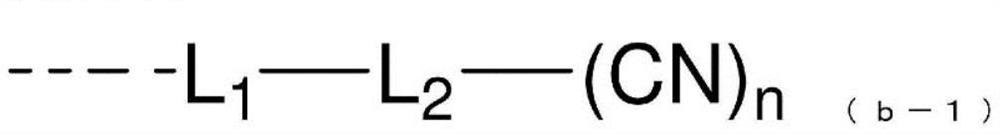Compound having benzotriazole ring structure and organic electroluminescence element
A technology of benzotriazole rings and compounds, which is applied in the fields of compounds with benzotriazole ring structures and organic electroluminescent elements, and can solve the problems of inability to fully exert functions, insufficient hole blocking functions, and glass transition Low temperature and other problems, to achieve the effect of excellent hole blocking ability, good injection characteristics, and improved luminous brightness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0144]
[0145] Charge 2-(4-chloro-phenyl)-5-(9,9'-spirobis[9H]fluoren-2-yl)-2H-benzotriazole in the reaction vessel: 9.0 g, 4-cyano Base-phenylboronic acid: 2.9g, tris(dibenzylideneacetone) dipalladium (0): 0.5g, tricyclohexylphosphine: 0.2g, tripotassium phosphate: 10.5g, in 1,4-dioxane, h 2 O mixed solvent under reflux and stirred overnight. After natural cooling, add ethyl acetate / H 2 O, the organic layer taken out by extraction and liquid separation was concentrated. The obtained concentrate was purified by column chromatography (carrier: silica gel, eluent: dichloromethane / n-heptane) to obtain 2-(4'-cyano-biphenyl-4-yl)-5- 8.1 g of (9,9'-spirobis[9H]fluoren-2-yl)-2H-benzotriazole (compound-2) yellow powder (yield: 80%).
[0146] [chemical 12]
[0147]
[0148] The structure of the obtained yellow powder was identified using NMR.
[0149] use 1 H-NMR (CDCl 3 ) detected the following 26 hydrogen signals.
[0150] δ(ppm)=8.46(2H), 7.97(2H), 7.94(1H), 7.90(3H),...
Embodiment 2
[0152]
[0153] Put 2-(4-bromophenyl)-5,6-dichloro-benzotriazole in the reaction vessel: 20.0g, 4'-(4,4,5,5-tetramethyl-[1, 3,2] dioxaborolan-2-yl)biphenyl-4-carbonitrile: 18.7g, toluene: 210mL, ethanol: 70mL, then, add potassium carbonate: 9.7g dissolved in H 2 O: an aqueous solution in 70 mL, nitrogen gas was passed through while irradiating ultrasonic waves for 30 minutes. Add tetrakis(triphenylphosphine)palladium: 1.7 g to the solution blown with nitrogen, and stir under reflux overnight under heating. Cool naturally after stirring, add methanol, H 2 O, carry out dispersion washing and filtration, obtain crude product. 2-(4-cyano-[1,1',4',1"]terphenyl-4"-yl)-5,6-bis was obtained by dispersing and washing the obtained crude product with acetone solvent 21.8 g (yield: 85%) of golden yellow (yamabuki color) powder of chloro-benzotriazole.
[0154] [chemical 13]
[0155]
[0156] For the obtained golden yellow powder, NMR was used to identify the structure.
[0157]...
Embodiment 3
[0166]
[0167] Charge 2-(4-cyano-[1,1',4',1"]terphenyl-4"-yl)-5,6-dichloro-benzotriazole in the reaction vessel: 5.0g, 1-Naphthylboronic acid: 6.2g, tris(dibenzylideneacetone) dipalladium (0): 0.5g, tricyclohexylphosphine: 0.6g, tripotassium phosphate: 14.4g, in 1,4-dioxane, H 2 O mixed solvent under reflux and stirred overnight. After natural cooling, add H into the system 2 O, carry out dispersion washing and filtration, obtain crude product. 2-(4-Cyano-[1,1',4',1"]terphenyl-4"-yl) was obtained by crystallizing the obtained crude product with dichlorobenzene / acetone mixed solvent - 4.6 g (yield: 65%) of white powder of 5,6-di(naphthalen-1-yl)-benzotriazole (compound-44).
[0168] [chemical 15]
[0169]
[0170] For the obtained white powder, the structure was identified using NMR.
[0171] use 1 H-NMR (CDCl 3 ) detected the following 28 hydrogen signals.
[0172] δ(ppm)=8.58(2H), 8.14(2H), 7.94-7.73(13H), 7.60(3H), 7.46(2H), 7.28(3H), 7.11(1H), 7.01(2H)
PUM
| Property | Measurement | Unit |
|---|---|---|
| electron work function | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com