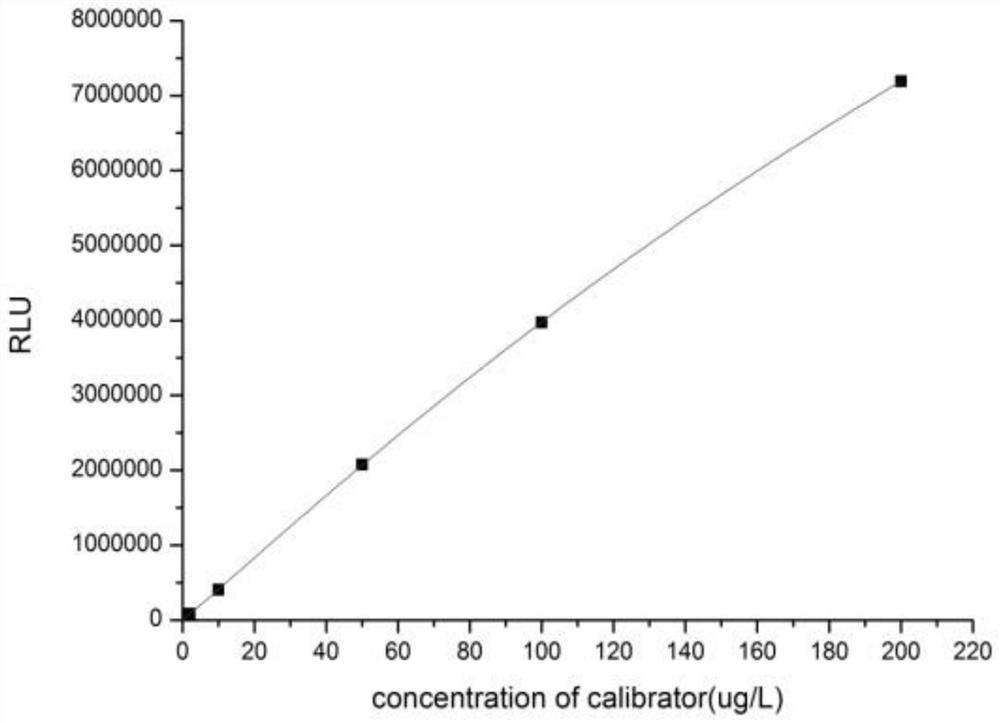
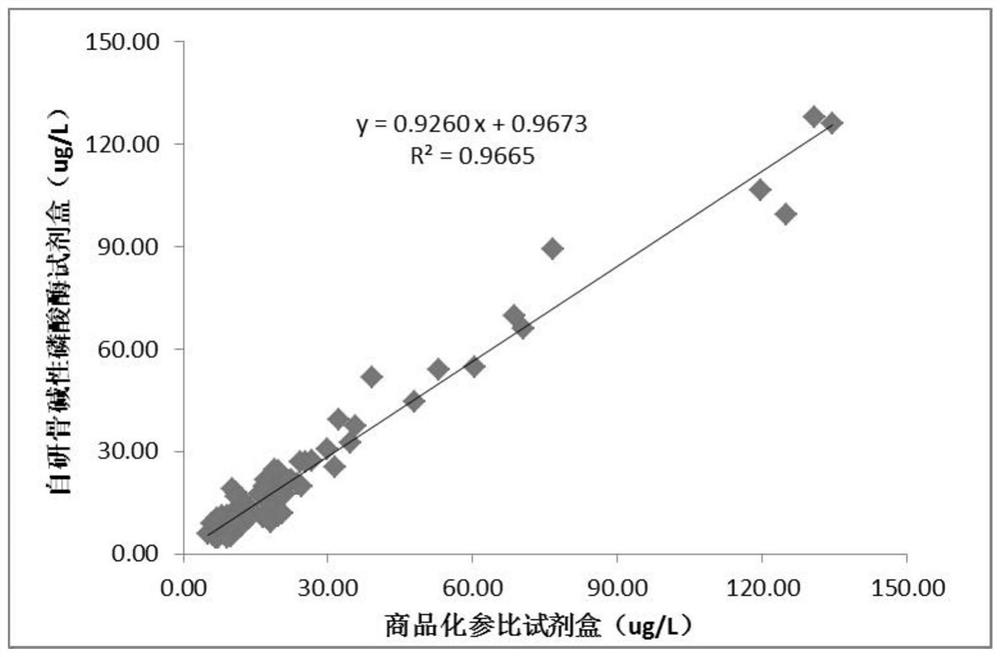
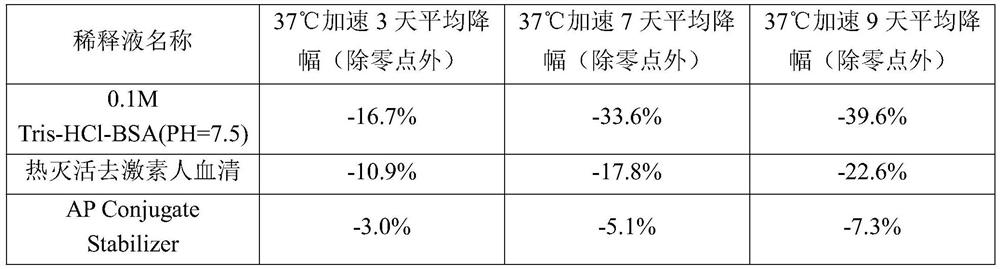
Magnetic particle chemiluminescence detection kit for determining content of human bone-derived alkaline phosphatase
A phosphatase and kit technology, applied in chemiluminescence/bioluminescence, microbial determination/inspection, biological testing, etc., can solve the problem of high false value of bone-derived alkaline phosphatase measurement, difficult automation, and measurement value. problems such as large variation, to achieve the effect of improving stability, simplifying production processes and procedures, and improving accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0061] A method of preparing a magnetic particulate chemiluminescence detection tester for determining human serum osteogenous alkaline phosphatase content, including the following steps:
[0062] Formulated R1 reagent: 1) Formulated according to the component content of the hepatyl alkaline phosphatase monoclonal antibody according to the R1 reagent buffer, after adjusting the pH to the designated value, add a hepatic alkaline phosphatase monoclonal antibody to form R1 Buffer of the reagent; 2) Preparation of osteocyanate fluorescein-labeled osteogeneous alkaline phosphatase monoclonal antibodies; 3) R1 with osteoplastic alkaline phosphatase monoclonal antibodies labeled isothiocyanate The buffer of the reagent is diluted.
[0063] Formulated magnetic separation reagent: 1) Substrate by magnetic particulate buffer component; 2) Preparation of magnetic particles of antibodithiosocyanate polyclonal antibody coated; 3) Buffer magnetic particles Dilution.
[0064] Formulated calibrat...
Embodiment 1
[0075] Example 1 A magnetic particulate chemiluminescence detection kit for determining a human boneborid alkaline phosphatase protein content includes a R1 reagent, magnetic separation reagent, a caliber, a quality control series, and a substrate fluid.
[0076] In the present embodiment, R1 reagents include: 1) R1 antibody: isothiocyanate fluorescene (FITC) labeled boneborne alkaline phosphatase monoclonal antibody, concentration of 0.8 μg / mL. 2) Buffer: Tris, concentration of 9.08 g / L; sodium concentration of 1.0 g / L; sodium chloride, concentration of 8.5 g / L, bovine serum albumin, concentration of 5.0g / L; liver source Single alkaline phosphatase monoclonal antibody, concentration of 1.0 μg / ml; the rest is deionized water. The buffer pH of the R1 reagent is preferably 7.5.
[0077] In this embodiment, the magnetic separation reagent comprises: 1) magnetic particles: anti-osteocyanate fluorescein (FITC) polyclonal antibody coated magnetic fine particles, concentratio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com