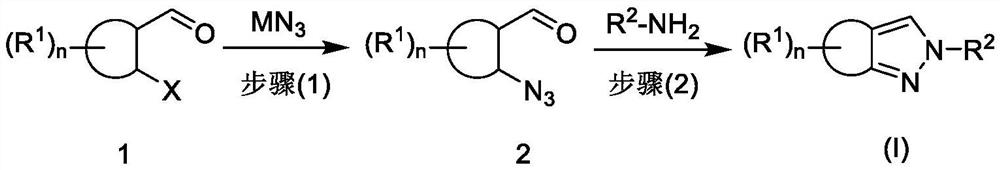
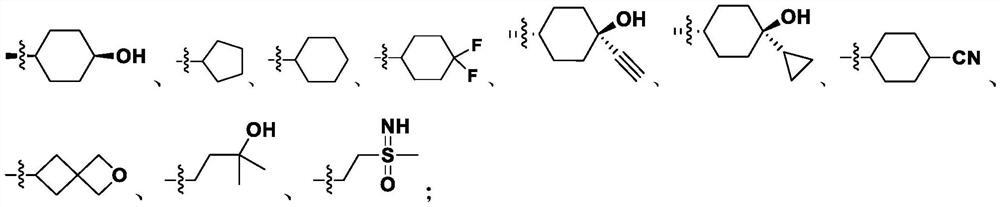
Preparation method of fused pyrazole compound
A technology for compounds and derivatives, applied in the field of organic synthesis, can solve the problems of difficult process production, poor selectivity, cumbersome operation, etc., and achieve the effects of improving yield and economy, avoiding safety risks, and having strong practicability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] Example 1: Preparation of 2-[3-(6-methoxy-5-nitro-indazol-2-yl)-cyclobutyl]-trans-propyl-2-alcohol
[0101]
[0102] 2-Fluoro-4-methoxy-5-nitrobenzaldehyde (10.00g) and sodium azide (3.60g) were sequentially added to methanol (100mL) solution, and the reaction mixture was stirred at 20-30°C for 16 hours. Without further treatment, the reaction mixture was a methanol solution of 2-azido-4-methoxy-5-nitrobenzaldehyde (50.00 mmol, based on a yield of 100%). To the above methanol solution of 2-azido-4-methoxy-5-nitrobenzaldehyde was added 2-(3-amino-cyclobutyl)-trans-propyl-2-ol (4.66 g) and concentrated Sulfuric acid (5.00g, 98%), keep the system reflux for 3 hours, the reaction temperature is 72-77 ° C, add water (40mL) to the reaction system, concentrate the mixed solution to a certain volume (40mL) under vacuum, after cooling, filter, The filter cake was dried to obtain 2-[3-(6-methoxy-5-nitro-indazol-2-yl)-cyclobutyl]-trans-propyl-2-alcohol (10.80g, two steps Yiel...
Embodiment 2
[0103] Example 2: Preparation of 2-[3-(6-methoxy-5-amino-indazol-2-yl)-cyclobutyl]-trans-propyl-2-alcohol
[0104]
[0105] Add 2-[3-(6-methoxy-5-nitro-indazol-2-yl)-cyclobutyl]-trans-propyl-2-ol (10.00g) to absolute ethanol (100mL) , under the protection of nitrogen, add palladium carbon (0.50g, anhydrous, 10% load rate), then replace with hydrogen, stir at 25-30°C for 3 hours under the pressure of a hydrogen balloon (1 atmosphere), add diatomaceous earth and filter, The filtrate was concentrated to give 2-[3-(6-methoxy-5-amino-indazol-2-yl)-cyclobutyl]-trans-propyl-2-ol (8.73g, yield: 96.1%) . 1 H NMR (400MHz, DMSO): δ7.90(s,1H),6.87(s,1H),6.62(s,1H),4.87(p,J=7.31Hz,1H),4.58(s,2H), 4.37(s,1H),3.82(s,3H),2.48(dd,J=16.43,8.83Hz,4H),2.44-2.32(m,1H),1.18-1.02(m,6H); LCMS m / z =276[M+H] + .
Embodiment 3
[0106] Example 3: 6-Trifluoromethyl-pyridine-2-carboxylic acid {2-[3'-(1-hydroxy-1-methyl-ethyl)-cyclobutyl]-6-methoxy-2 Preparation of Hydrogen-Indazol-5-yl}-amides
[0107]
[0108] 2-[3-(6-Methoxy-5-amino-indazol-2-yl)-cyclobutyl]-trans-propyl-2-ol (8.25 g) was added to tetrahydrofuran (60 mL), then Add 5-trifluoromethyl-2-pyridinecarboxylic acid (5.73g), 2-(7-azabenzotriazole)-N,N,N',N'-tetramethyluronium hexafluorophosphate in sequence (12.55g) and N,N-diisopropylethylamine (4.27g), the reaction mixture was stirred at room temperature for 4 hours, water (120mL) was added, filtered, and the filter cake was dried to obtain compound 1 (11.49g, Yield: 85.4%). 1 HNMR (400MHz, CDCl 3 ): δ10.71(s,1H),8.81(s,1H),8.50(d,J=8.0Hz,1H),8.11(t,J=8.0Hz,1H),7.89(s,1H),7.86 (d,J=6.8Hz,1H),7.11(s,1H),5.05-4.99(m,1H),4.03(s,3H),2.79-2.72(m,2H),2.67-2.60(m,3H ), 1.34(s,1H), 1.25(s,6H); LCMS m / z=449.2[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com