Method for preparing ruthenium trichloride hydrate by adopting ruthenium-containing wastewater
A technology of ruthenium trichloride hydrate and ruthenium trichloride, which is applied in the field of preparing ruthenium trichloride hydrate, can solve the problems of long reaction time, low ruthenium enrichment rate, difficult product quality, etc., and achieve improved absorption rate and Reaction efficiency, increased gas-liquid contact area, and effective recycling effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
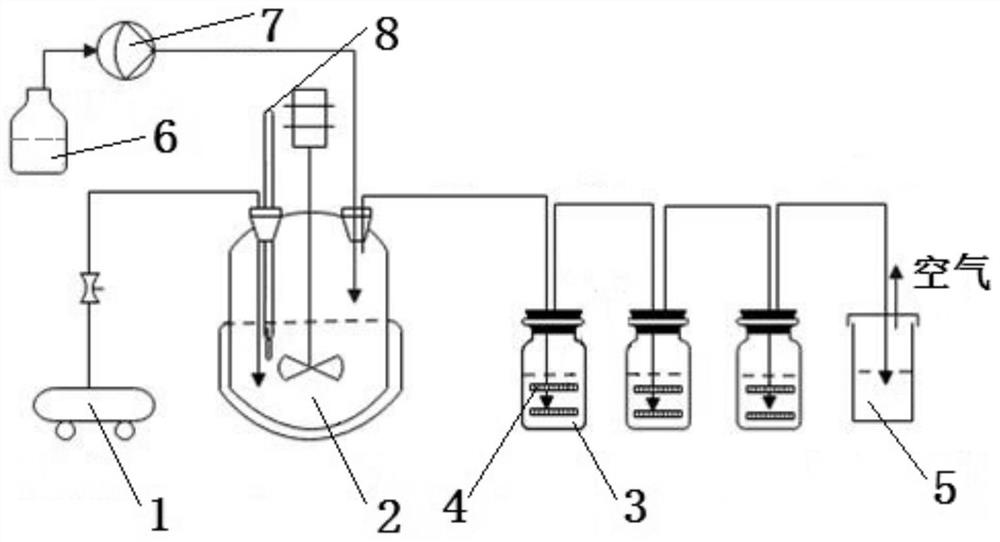
[0048] (1) According to figure 1 Build the reaction device as shown, and check the air tightness of the device (two absorption devices are installed, and two layers of microporous sand core glass plates are installed in each absorption device, and the pore diameter of the microporous sand core is 1 μm).
[0049] (2) Accurately weigh 30 kg of ruthenium-containing spent catalyst solution (brown black, use ICP-OES to detect Ru content 500 ppm), add it to the distillation reactor, weigh 200 g of hypochlorous acid (mass concentration 20%) and add it to the oxidant storage bottle ;
[0050] (3) 3L of HCl with a mass concentration of 15% is respectively configured in the 1st to 2nd-stage absorption bottles, and 30g of absolute ethanol is added;
[0051] (4) Turn on the gas cylinder switch and set the gas purge rate to 0.4L / min;
[0052] (5) Turn on the dropping funnel switch and set the pump speed to 10mL / min;
[0053] (6) Oxidation reaction at 50°C, the liquid in the primary abso...
Embodiment 2
[0064] (1) According to figure 1 Build the reaction device as shown, and check the air tightness of the device (there are 3 absorption devices, and each absorption device is equipped with 2 layers of glass plates with microporous sand cores, and the pore diameter of the microporous sand cores is 1 μm).
[0065] (2) Accurately take by weighing 30kg of spent catalyst solution containing ruthenium (brown yellow, after detecting Ru content 30ppm), add in the distillation reactor, take by weighing 300g sodium chlorate (available chlorine content 10%), add in the oxidant storage bottle;
[0066] (3) Prepare 1 L of HCl absorption solution with a mass concentration of 5% in 1-3 absorption bottles, and add 10 g of absolute ethanol;
[0067] (4) Turn on the gas cylinder switch and set the gas purge rate to 0.4L / min;
[0068] (5) Turn on the dropping funnel switch and set the pump speed to 5mL / min;
[0069] (6) Oxidation reaction at 50°C, the liquid in the first-level absorption bottle...
Embodiment 3
[0073] (1) According to figure 1 Build the reaction device as shown, and check the air tightness of the device (two absorption devices are installed, and 5 layers of microporous sand core glass plates are installed in each absorption device, and the pore diameter of the microporous sand core is 100 μm).
[0074] (2) Accurately weigh 30 kg of ruthenium-containing spent catalyst solution (brownish yellow, 30 ppm of Ru content after detection), add it to the distillation reactor, weigh 300 g of hydrogen peroxide (mass concentration 30%), and add it to the oxidant storage bottle;
[0075] (3) Prepare 1 L of HCl absorption solution with a mass concentration of 5% in 1-2 absorption bottles, and add 10 g of absolute ethanol;
[0076] (4) Turn on the gas cylinder switch and set the gas purge rate to 1.0L / min;
[0077] (5) Turn on the dropping funnel switch and set the pump speed to 5mL / min;
[0078] (6) Oxidation reaction at 30°C, the liquid in the primary absorption bottle turns li...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com