Free radical initiator, preparation method thereof, light-emitting free radical polymer and preparation method of light-emitting free radical polymer
A technology of free radicals and initiators, applied in luminescent materials, chemical instruments and methods, organic chemistry, etc., can solve the problems of unreported free radical fluorescent polymers, difficult control of free radical content and spatial distribution, and achieve easy processing, Good mechanical properties, good film-forming performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0072] The application also provides a preparation method of the free radical initiator, comprising:
[0073] a first reaction of the first compound with 3,4-dihydro-2H-pyran (THP) to obtain a second compound, and then a second reaction of the second compound with a trityl compound to obtain a third compound;
[0074] performing a de-THP protection reaction on the third compound to obtain a fourth compound, and then performing a dehydrogenation reaction on the fourth compound to obtain the free radical initiator;
[0075] The structural formula of the first compound is:
[0076]
[0077] The structural formula of the second compound is:
[0078]
[0079] The general structural formula of the third compound is:
[0080]
[0081] The general structural formula of the fourth compound is:
[0082]
[0083] Wherein, Y is a trityl group.
[0084] In order to better illustrate the above-mentioned trityl groups, an example is given: for example, when Y is a trichlorotr...
Embodiment 1
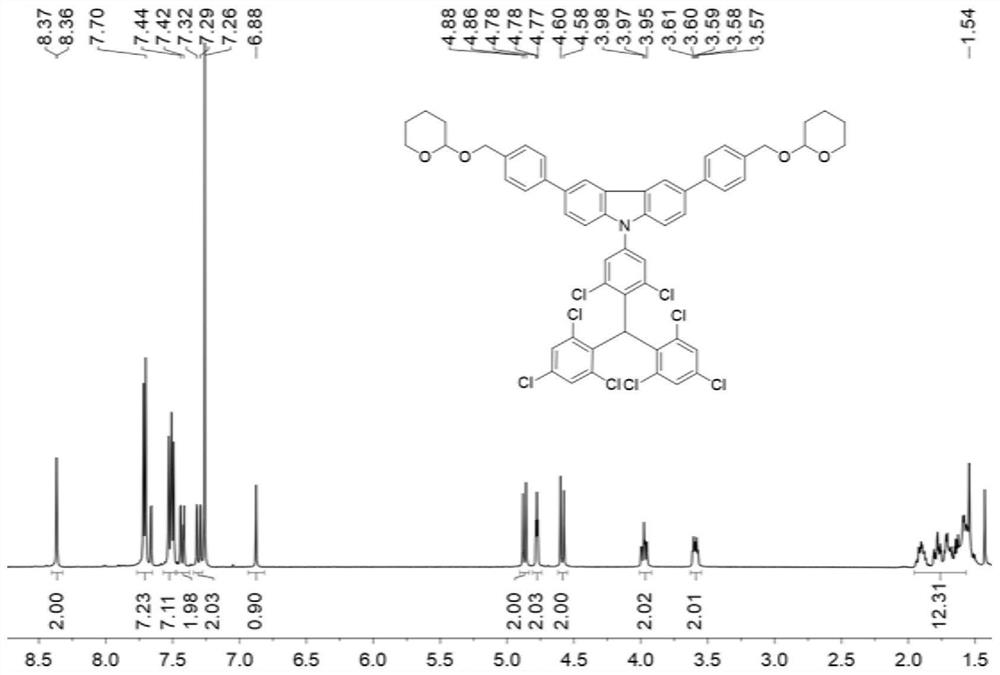
[0108] The present embodiment provides a kind of free radical initiator, and its structural formula is:
[0109]
[0110] Its preparation method is as follows:
[0111] step 1:
[0112]
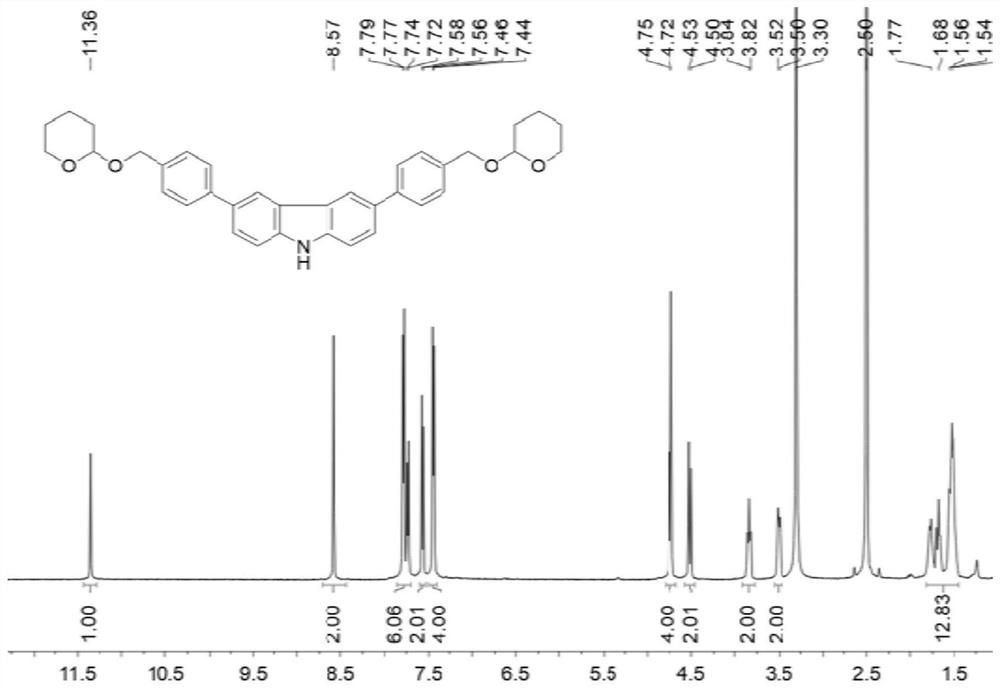
[0113] Into a 50 mL round bottom flask was added the first compound CZ-OH (3.80 g, 10 mmol), p-toluenesulfonic acid monohydrate (1.52 g, 8 mmol) and 20 mL of tetrahydrofuran. 3,4-Dihydro-2H-pyran (2.74 mL, 30 mmol) was slowly added and stirred at room temperature for 1 hour. After the reaction was completed, saturated aqueous sodium bicarbonate solution was added for neutralization. The organic layer was washed with saturated brine and dried over anhydrous magnesium sulfate. The solvent was removed by rotary evaporation. With petroleum ether: ethyl acetate=8:1 (volume ratio) as eluent, purify by column chromatography, obtain 2.74g (5mmol) white solid product, productive rate 50%, be the second compound (in the above equation Compound 1), its hydrogen spectrum is as figure 1 shown....
Embodiment 2
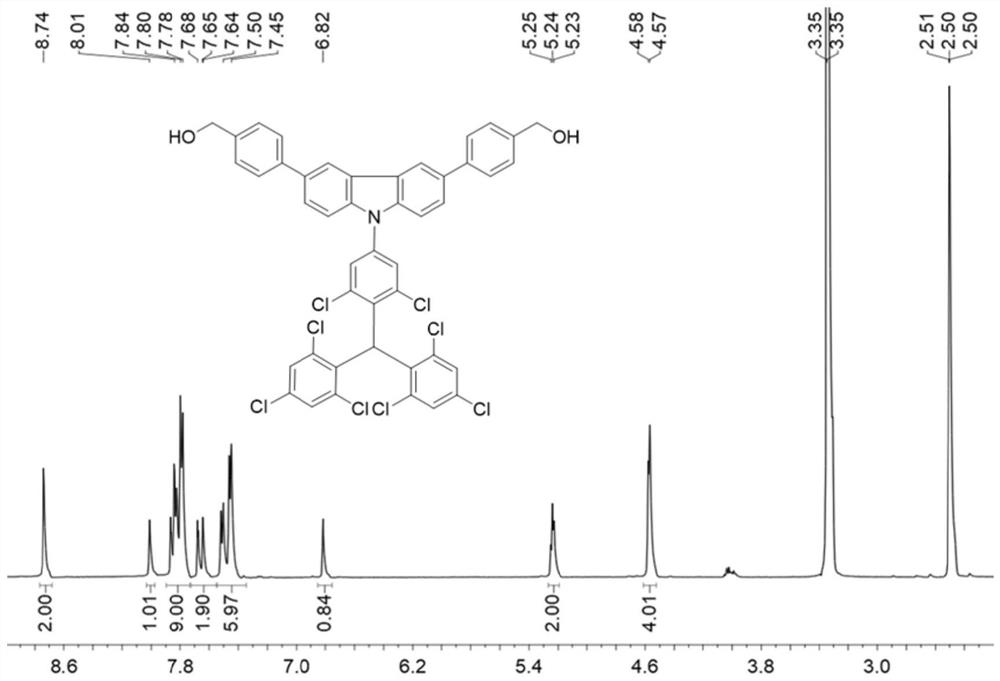
[0125] This embodiment provides a light-emitting free radical polymer, the general structural formula of which is:
[0126]
[0127] This embodiment also provides a preparation method of the above-mentioned light-emitting radical polymer, specifically as follows:
[0128] Under the protection of nitrogen atmosphere, add the free radical initiator (27mg, 0.03mmol) that embodiment 1 obtains in the Schlenk bottle after toasting through 20mL, catalyst stannous octoate Sn(Oct)2 (0.01mmol), monomer δ-valerolactone (300mg, 3mmol) and 5mL toluene, react at 130°C for 6 hours. The reaction system was cooled to room temperature, precipitated with methanol, and centrifuged. The solid was dissolved in a small amount of dichloromethane, and precipitated three times with methanol. The solid obtained by centrifugation was vacuum-dried to obtain a light green polymer with a yield of 70%. The relative number average molecular weight (n) is 9.7kg / mol, and the molecular weight distribution (w / ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Relative number average molecular weight | aaaaa | aaaaa |
| Relative number average molecular weight | aaaaa | aaaaa |
| Relative number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com