Polymer semiconductor containing quinoid-donor-receptor unit, and preparation method and application thereof
An acceptor unit and polymer technology, applied in the field of polymer semiconductors and their preparation, can solve the problems of lowering the lowest occupied orbit, blurring boundaries, affecting the device performance of polymers, etc., achieving high yield, simple synthesis path, The effect of great development prospects and potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
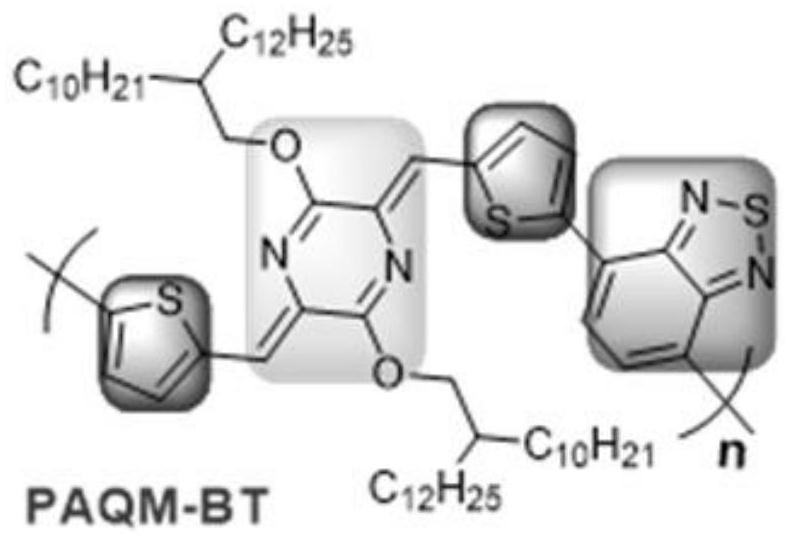
[0032]
[0033] The synthesis path is as above
[0034] 1) Under inert gas conditions, (3Z, 6Z)-3,6-bis((5-bromothiophen-2-yl)methylene)piperazine-2,5-dione (1.6mmol, 1eq) , potassium carbonate (8.0mmol, 5eq), alkyl bromide (6.4mmol, 4eq) and N,N-dimethylformamide (15mL) were added to the reaction flask, heated to 90-110°C, reacted for 2 hours, and cooled to After room temperature, the reaction mixture was suction-filtered, and the filtrate was concentrated by rotary evaporation under reduced pressure, and subjected to silica gel column chromatography (hexane / dichloromethane ratio of 9:1 to 3:1), spin-dried and then used anhydrous Ethanol was recrystallized to obtain the desired compound 1 as an orange solid;
[0035] 2) Under inert gas conditions, compound 1 (600mg, 0.53mmol, 1eq) was dissolved in dry tetrahydrofuran (40mL) solution, cooled to -78°C, and n-butyllithium (0.83mL, 1.6M, 2.5 eq), after stirring the reaction for 1-2 hours, trimethyltin chloride (1.43mL, 1M, 2...
Embodiment 2
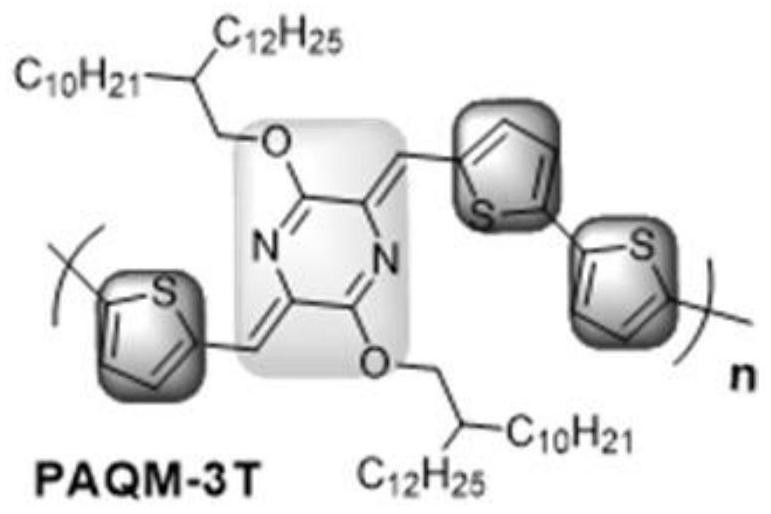
[0039] The synthesis route of this example is the same as that of Example 1, the difference is that the raw materials for preparing Compound 1 are different, and the compound 1 synthesized has a structural formula of The structural formula of compound 3 is
Embodiment 3
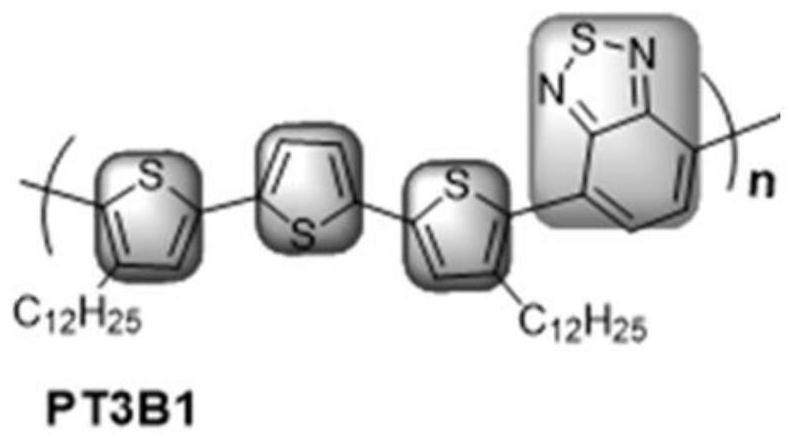
[0041] The synthesis route of this example is the same as that of Example 1, the difference is that the raw materials for preparing Compound 1 are different, and the compound 1 synthesized has a structural formula of The structural formula of compound 3 is
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com