Preparation process of cefotaxime sodium for injection
A preparation technology of cefotaxime sodium, which is applied in the field of medicine and chemical industry, can solve the problems of low product purity, impurities, and poor stability, and achieve uniform crystal particle size, reduce degradation or side reactions, and improve stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
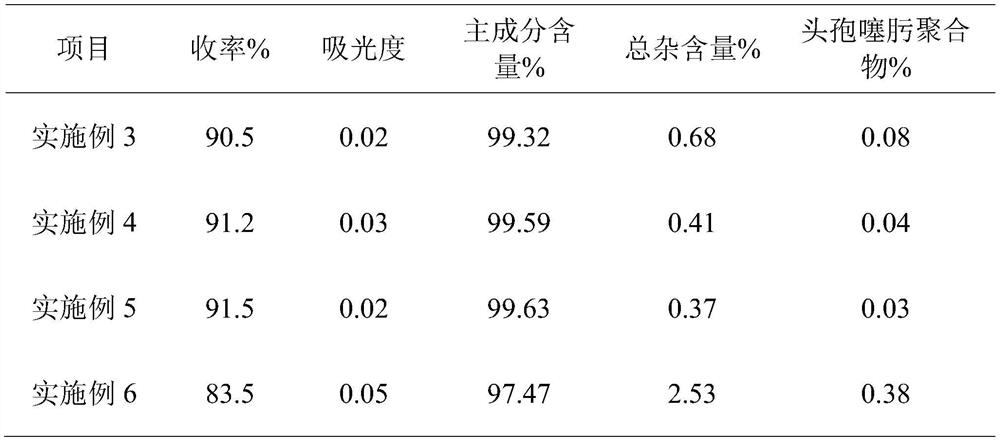
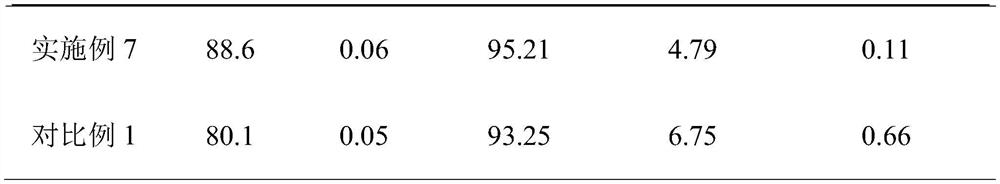
Embodiment 1
[0029] A preparation process of cefotaxime sodium for injection, comprising the following steps:
[0030] S1. Preparation of mixed solvent: take ethanol and n-propanol and mix them at a volume ratio of 7:1 to obtain a composite organic solvent, and add water to make a mixed solvent with an organic solvent volume fraction of 50% for later use;
[0031] S2. Under the condition of controlling the temperature at 25° C., according to the solid-liquid ratio of 50 mg / mL, sodium acetate is added to the mixed solvent prepared in step 1 to mix and dissolve to obtain a sodium acetate solution;
[0032] S3, under the stirring state of 110r / min, add cefotaxime acid step by step to the sodium acetate solution in a mass ratio of 0.3:1, the first addition of cefotaxime acid is 1 / 5 of the prescription amount of cefotaxime acid, Then add 1 / 10 of the prescription amount of cefotaxime acid in equal increments each time, with an interval of 100 seconds, keep stirring at a constant temperature of 4...
Embodiment 2
[0037] A preparation process of cefotaxime sodium for injection, comprising the following steps:
[0038] S1. Preparation of mixed solvent: take ethanol and n-propanol and mix them in a volume ratio of 7:2 to obtain a composite organic solvent, and add water to make a mixed solvent with an organic solvent volume fraction of 70% for later use;
[0039] S2. Under the condition of controlling the temperature at 35° C., according to the solid-liquid ratio of 80 mg / mL, sodium acetate is added to the mixed solvent prepared in step 1 to mix and dissolve to obtain a sodium acetate solution;
[0040] S3, under the stirring state of 130r / min, add cefotaxime acid step by step to the sodium acetate solution in a mass ratio of 0.4:1, the first addition of cefotaxime acid is 1 / 5 of the prescription amount of cefotaxime acid, Then add 1 / 15 of the prescription amount of cefotaxime acid in equal increments each time, with an interval of 120 seconds, keep stirring at a constant temperature of 5...
Embodiment 3
[0045]A preparation process of cefotaxime sodium for injection, comprising the following steps:
[0046] S1. Preparation of mixed solvent: take ethanol and n-propanol and mix them in a volume ratio of 7:2 to obtain a composite organic solvent, and add water to make a mixed solvent with an organic solvent volume fraction of 55% for later use;
[0047] S2. Under the condition of controlling the temperature at 30° C., according to the solid-liquid ratio of 65 mg / mL, sodium acetate is added to the mixed solvent prepared in step 1 to mix and dissolve to obtain a sodium acetate solution;
[0048] S3, under the stirring state of 120r / min, add cefotaxime acid step by step to the sodium acetate solution in a mass ratio of 0.35:1, the first addition of cefotaxime acid is 1 / 5 of the prescription amount of cefotaxime acid, Then add 1 / 10 of the prescription amount of cefotaxime acid each time in an equal increment manner, with an interval of 120 seconds, and keep stirring at a constant tem...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com