Atomization method of treprostinil aerosol inhalant for treating pulmonary arterial hypertension
A technology for treprostinil and pulmonary arterial hypertension, which is applied in nebulizers for treatment, aerosol delivery, spray delivery, etc. It can solve the problems of affecting patients' inhalation experience, affecting drug efficacy, and equipment cannot be placed flat or dumped. , to achieve the effect of convenient daily carrying and use, increasing medication compliance, and less medication dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Accurately weigh 120 mg of treprostinil and put it in a volumetric flask, add 10 ml of methanol, and shake to dissolve. Using a high-precision ultrasonic atomization coating machine, the drug solution is coated on the foil array of the drug inhaler, and the coating amount is 30 μg / cm 2 ,dry. When in use, the patient inhales air, and the heating controller in the inhalation system will give the foil a pulse current of 3 volts for 0.3 seconds to rapidly heat the foil to 300°C and vaporize treprostinil to form an aerosol, which can be inhaled The airflow diversion controller in the device controls the airflow above the foil to be 2-7L / min, and about 6 μg of treprostinil aerosol is brought into the human body.
Embodiment 2
[0039] Accurately weigh 300 mg of treprostinil diethanolamine salt, put it in a volumetric flask, add 10 ml of absolute ethanol, and shake to dissolve. Using a high-precision ultrasonic atomization coating machine, the drug solution is coated on the foil array of the drug inhaler, and the coating amount is 90 μg / cm 2 ,dry. When in use, the patient inhales air, and the heating controller in the inhalation system will give the foil a pulse current of 4.5 volts for 0.5 seconds to rapidly heat the foil to 250°C and vaporize treprostinil diethanolamine salt to form gas. For the aerosol, the airflow diversion controller in the inhaler controls the airflow above the foil to be 2-7L / min, and about 15 μg of treprostinil diethanolamine salt aerosol is brought into the human body.
Embodiment 3
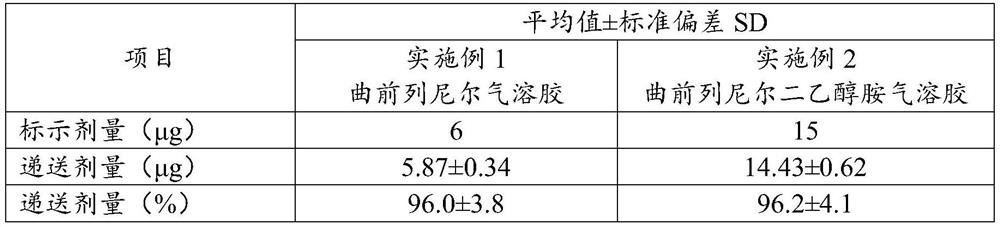
[0040] Example 3 Delivery Dose Determination
[0041] According to Chinese Pharmacopoeia delivery dose uniformity requirement, adopt DUSA tube (dosage unit sampling apparatus, unit dose sampling device) method, the treprostinil aerosol made in embodiment 1 and the treprostinil diethanolamine made in embodiment 2 The delivered dose of the aerosol was determined.
[0042] Connect the suction port of the aerosol system to the DUSA tube, turn on the vacuum pump, and adjust the throttle valve of the flow meter to make the flow rate reach 28.3L / min (±5%). The aerosol system uses a suitable rubber interface to connect with the DUSA tube equipped with a filter membrane to ensure that the connection is sealed. Spray the sample into the DUSA tube once, add methanol and sodium hydroxide solution with a pipette gun, vortex for 30s, and sonicate for 10min. The supernatant was taken as the test solution, and the operation was repeated 10 times. It was measured by high performance liquid ch...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com