Non-fullerene receptor with collaborative assembly characteristic as well as preparation method and application of non-fullerene receptor
A non-fullerene acceptor and cooperative assembly technology, applied in semiconductor/solid-state device manufacturing, electrical solid-state devices, semiconductor devices, etc., can solve the problem that non-fullerene acceptors cannot obtain high performance, and avoid phase separation Effects of defects, cost reduction, and prolongation of crystallization time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
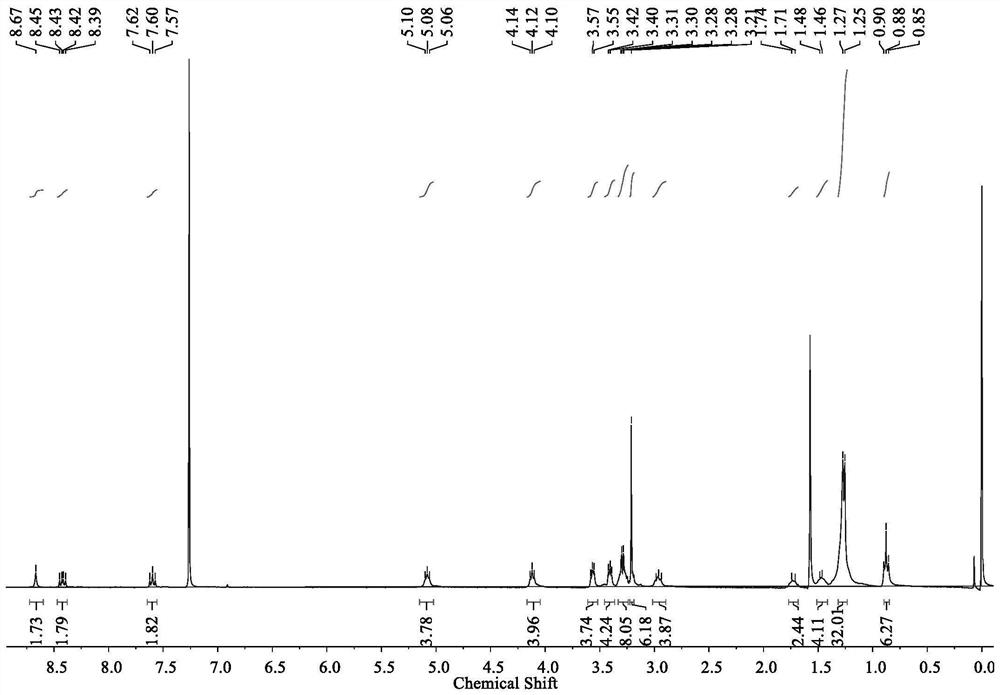
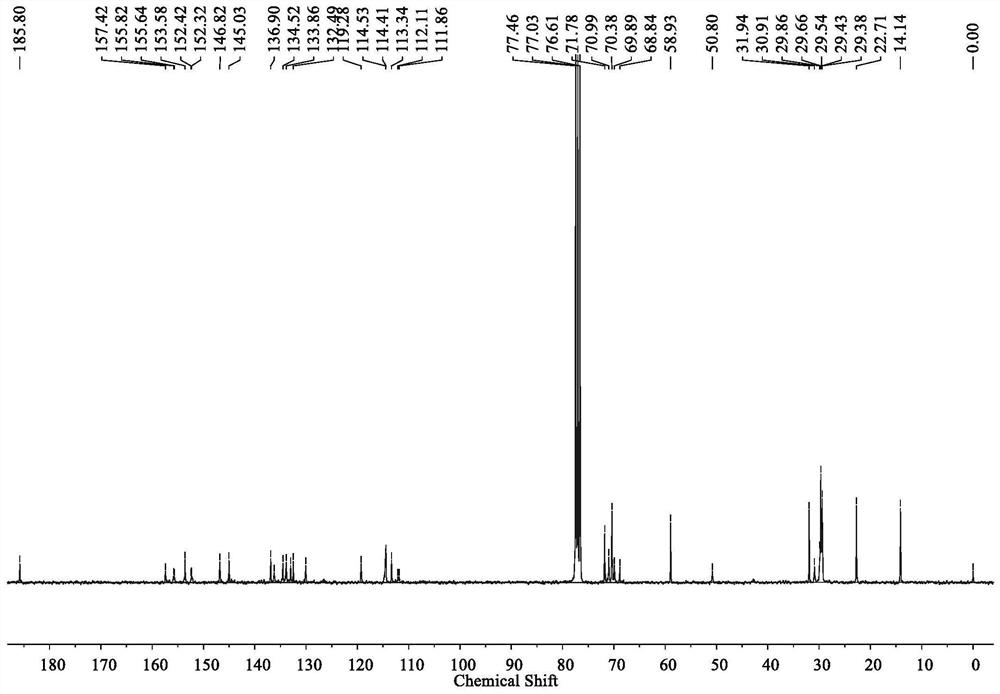
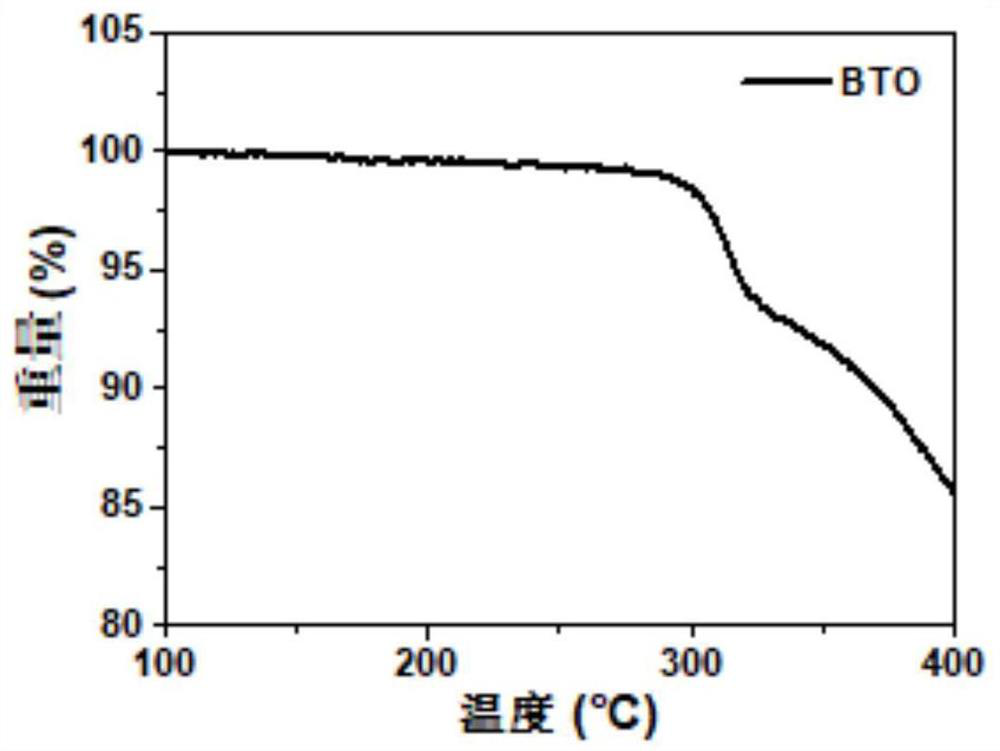
[0058] Example 1 The structural formula of the non-fullerene acceptor material (BTO) with cooperative assembly performance is:
[0059]
[0060]
[0061] The preparation method of above-mentioned BTO is as follows:
[0062] 1 M sodium hydroxide solution (50 mL) was added to a solution of triethylene glycol monomethyl ether (16.41 g, 0.10 mol) in tetrahydrofuran (20 mL) under an ice-water bath, p-toluenesulfonyl chloride (19.64 g, 0.10 mol) of a saturated tetrahydrofuran solution was added dropwise to the reaction system, and stirred overnight at room temperature to obtain a colorless liquid 2-[2-(2-methoxyethoxy)ethoxy]ethyl 4-methylbenzenesulfonate;
[0063] Under nitrogen protection, 3,9-diundecyl-12,13-dihydro-[1,2,5]thiadiazolo[3,4-e]thieno[2'',3'': 4',5']thieno[2',3':4,5]pyrrolo[3,2-g]thieno[2',3':4,5]thieno[3,2-b] Indole (747.17 mg, 1 mmol) and tetrabutylammonium bisulfate (86.98 mg, 0.25 mmol) were dissolved in 20 mL of toluene, 0.50 M sodium hydroxide solution ...
Embodiment 2
[0070] Example 2 The structural formula of the non-fullerene acceptor material (BT-2OEG-4F) with cooperative assembly performance is:
[0071]
[0072]
[0073] The preparation method of above-mentioned BT-2OEG-4F is as follows:
[0074] Under nitrogen protection, 3,9-diundecyl-12,13-dihydro-[1,2,5]thiadiazolo[3,4-e]thieno[2'',3'': 4',5']thieno[2',3':4,5]pyrrolo[3,2-g]thieno[2',3':4,5]thieno[3,2-b] Indole (747.17 mg, 1 mmol) and tetrabutylammonium bisulfate (86.98 mg, 0.25 mmol) were dissolved in 20 mL of toluene, 0.50 M sodium hydroxide solution (7 mL) was added and stirred at room temperature for 15 min, then 4- 2-(2-Methoxyethoxy)ethoxy]ethyl toluenesulfonate (603.53 mg, 2.20 mmol) was added to the reaction system, and the system was stirred at 80°C for 12 h to obtain bright yellow solid 12 ,13-bis(2-(2-methoxyethoxy)ethoxy)ethyl)-3,9-diundecyl-12,13-dihydro-[1,2,5]thiadi Azolo[3,4-e]thieno[2'',3'':4',5']thieno[2',3':4,5]pyrrolo[3,2-g]thieno [2',3':4,5]thieno[3,2-...
Embodiment 3
[0078] Example 3 The structural formula of the non-fullerene acceptor material (BT-4OEG-4F) with cooperative assembly performance is:
[0079]
[0080]
[0081] The preparation method of above-mentioned BT-4OEG-4F is as follows:
[0082]Under nitrogen protection, 3,9-diundecyl-12,13-dihydro-[1,2,5]thiadiazolo[3,4-e]thieno[2'',3'': 4',5']thieno[2',3':4,5]pyrrolo[3,2-g]thieno[2',3':4,5]thieno[3,2-b] Indole (747.17 mg, 1 mmol) and tetrabutylammonium bisulfate (86.98 mg, 0.25 mmol) were dissolved in 20 mL of toluene, 0.50 M sodium hydroxide solution (7 mL) was added and stirred at room temperature for 15 min, then 4- 2-[2-[2-(2-methoxyethoxy)ethoxy]ethyl toluenesulfonate (800.80 mg, 2.20 mmol) was added to the reaction system, and the system was stirred at 80°C for 12 h , to give bright yellow solid 12,13-bis(2-(2-(2-(2-methoxyethoxy)ethoxy)ethyl)-3,9-diundecyl-12,13- Dihydro-[1,2,5]thiadiazolo[3,4-e]thieno[2'',3'':4',5']thieno[2',3':4,5 ]pyrrolo[3,2-g]thieno[2',3':4,5]t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com