Preparation method of isoxazoline anthelmintic fluralaner
A technology of isoxazolines and flurelana, applied in the field of chemistry or medicinal chemistry, can solve the problems of harsh conditions, high experimental operation requirements, high price, etc., and achieves mild reaction conditions, short synthetic routes, and easy operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] (1) Ring: 4- [3- (3,5-dichlorophenyl) -4,4,4-trifluorobutyl-2-enyloyl] -2-methyl-benzene in the 1L reaction bottle Ethyl formate was ethyl formate 43.1 g (0.1 mol) and hydroxylamine hydroxylamine (0.2 mol) of isoamate, acetate is 10.2 g (0.03 mol), and lithium hydroxide 6.0 g of hydroxide is added in batches under stirring (0.25) Mol) / water 30g, add 5 hours after 25-30 ° C, the reaction is completed, stationary, the upper organic layer is concentrated under reduced pressure, the temperature is 60 ° C, the degree of vacuum is -0.09 MPa, resulting in a condensate 46.5 g. HPLC detection was 98.1%.
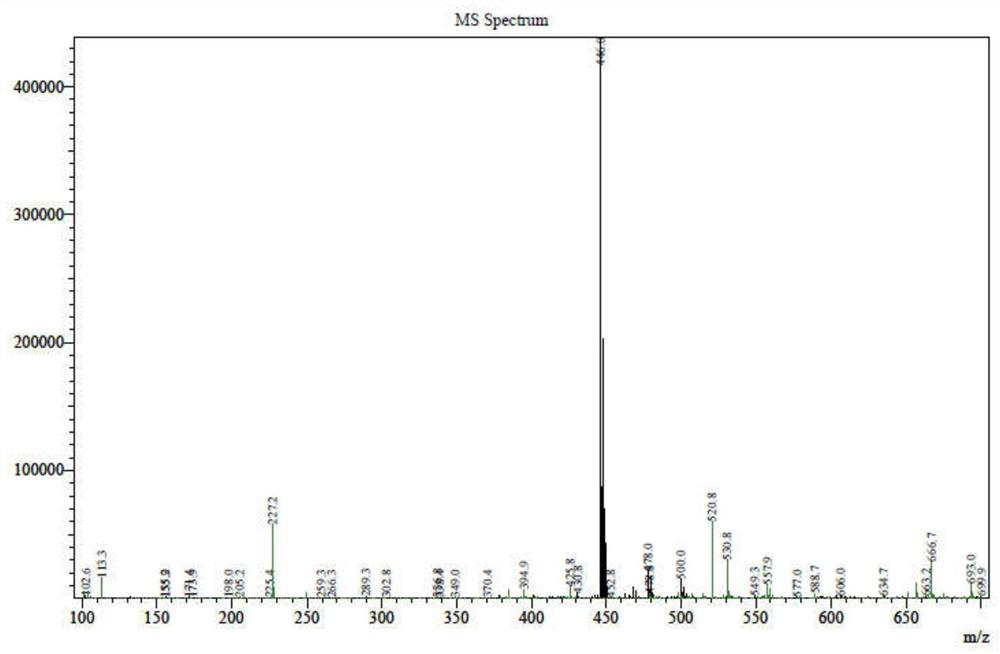
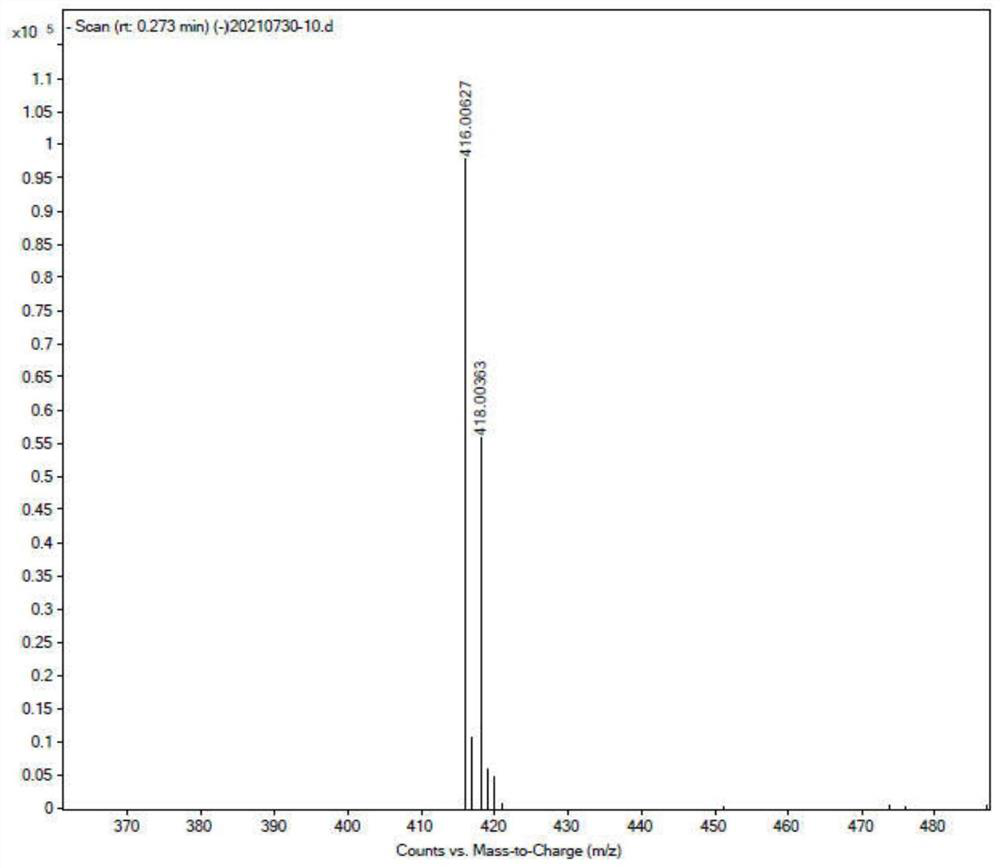
[0037] [M] = 446.0 (see figure 1 )
[0038] (2) Hydrolysis: The above condensate 46.5 g (0.1 mol) and 320 g of methanol, 20.2 g (0.25 mol) of ammonia were 80-65 ° C for 6 hours, and the temperature was 10-15 ° C. PH = 2-3 by phosphoric acid. Pumped filtration, water rinsing to neutral. It was dried at 50 ° C for 16 hours to give 39.5 g of hydrolyzate. Ring and hydrolysis two-step...
Embodiment 2
[0047] (1) Ring: 4- [3- (3,5-dichlorophenyl) -4,4,4-trifluorobutyl-2-enyloyl] -2-methyl-benzene in the 1L reaction bottle Ethyl formate was ethyl formate and 7.6 g (0.11 mol) of hydroxylamine hydroxylamine, butyl acetate 250g, benzyl triethyl ammonium chloride 1.14 g (0.005 mol), and potassium hydroxide was added batch with stirring (EtOAc) 0.15 mol) / water 20g, the addition of 25-30 ° C for 2 hours, the reaction is completed, stationary, the upper organic layer is concentrated under reduced pressure, the temperature is 60 ° C, the vacuum is -0.1MPa, resulting in a condensate 46.8 g. HPLC detection was 98.0%.
[0048] (2) Hydrolysis: The above-mentioned condensate 46.8 g (0.1 mol) and isopropyl alcohol 320g, 10.1 g (0.18 mol) / water 25g, temperature rise to 40-45 ° C for 4 hours, cool down 10 -15 ° C. PH = 1-2 was adjusted with 30% hydrochloride. Pumped filtration, water rinsing to neutral. 60 ° C is dried for 15 hours to give hydrolyzate 39.7 g. Ring and hydrolysis of two-step ...
Embodiment 3
[0051] (1) Ring: 4- [3- (3,5-dichlorophenyl) -4,4,4-trifluorobutyl-2-enyloyl] -2-methyl-benzene in the 1L reaction bottle Ethyl formate 43.1 g (0.1 mol) and hydroxylamine hydroxylamine (0.16 mol), methyl tert-butyl ether 280g, triethylmomethyl ammonium chloride 4.04 g (0.01 mol), stirred with batch of sodium carbonate under stirring 15.9 G (0.15 mol) / water 60g, the addition is completed at 25-30 ° C for 2 hours, the reaction is completed, stationary, the upper organic layer is concentrated under reduced pressure, the temperature is 40 ° C, the vacuum is -0.08MPa, resulting in a condensate 46.3 g. HPLC detection was 98.2%.
[0052] (2) Hydrolysis: The above-mentioned condensate 46.8 g (0.1 mol) and 460 g of methanol, 26.5 g (0.25 mol) / water 100g, temperature rise to 75-80 ° C for 4 hours, cooling 10-15 ° C . PH = 1-2 with formic acid. Pumped filtration, water rinsing to neutral. The heating was dried at 90 ° C for 10 hours to give 30 hydrolyzate 39.3 g. The ring combination and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com