Antibacterial hemostatic porous microsphere based on sodium alginate and nanocrystalline cellulose
A technology of nanocrystalline cellulose and sodium alginate, which is applied in the fields of application, medical science, surgery, etc., can solve the problems that affect the biocompatibility of microspheres, do not have antibacterial properties, and strong cytotoxicity, so as to prevent wound infection, Good biocompatibility, effect of promoting hemostasis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 1) Oil phase: Add different amounts of Span 80 (2%, 3%, 4%, 5%, 6%) to 90g of liquid paraffin, stir at 700rpm, 45°C for 1h;
[0032] 2) Water phase: Sodium alginate and cellulose nanocrystals (different mass ratios of the two in the water phase: 1:1, 2:1, 3:1, 4:1, 5:1 and the sum of the two accounted for Water phase mass ratio: 0.5%, 1.0%, 1.5%, 2.0%, 2.5%) were dissolved in 30ml deionized water.
[0033] 3) Add the water phase dropwise into the oil phase (the weight ratio of the water phase to the oil phase is 1:3), stir at 800rpm for 2h, then add 6ml of calcium chloride solution (20wt%) dropwise, and stir at 800rpm for 4h; Wash 2 times with n-hexane, absolute ethanol, and deionized water, centrifuge to remove the supernatant, and freeze-dry to obtain SA / NCC porous microspheres; 100 mg of freeze-dried SA / NCC porous microspheres are dropped into 20 ml polylysine solution (6 mg / ml), stirred at 200rpm for 24h, centrifuged and freeze-dried to obtain SA / NCC@PL porous mic...
Embodiment 2
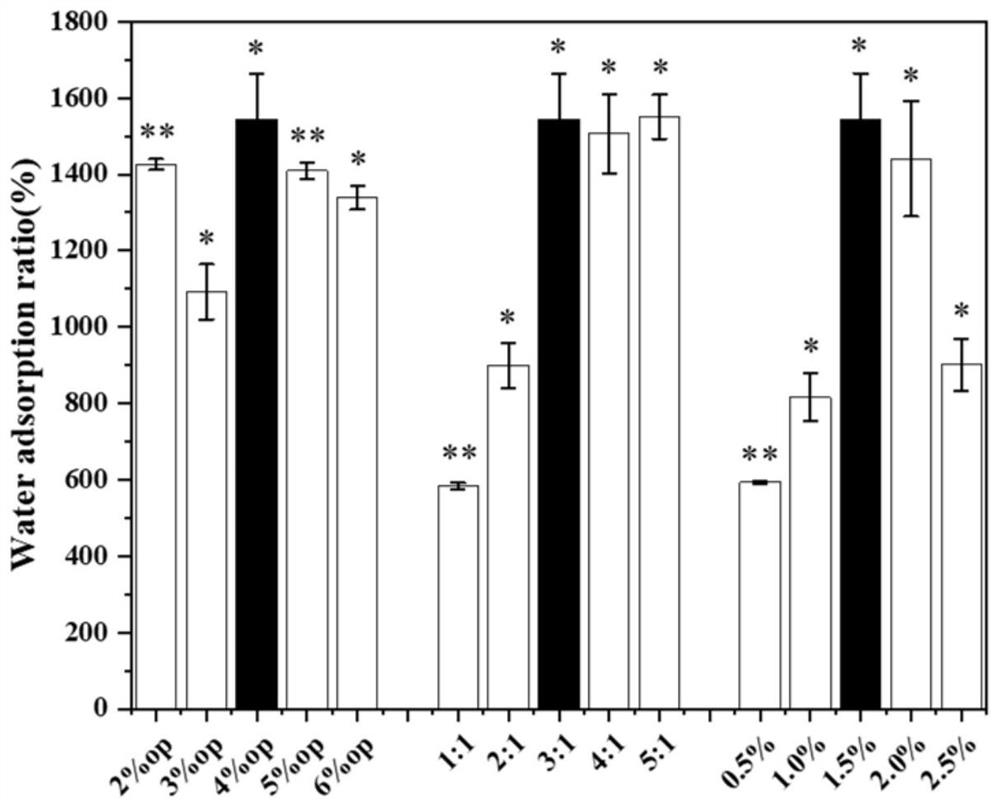
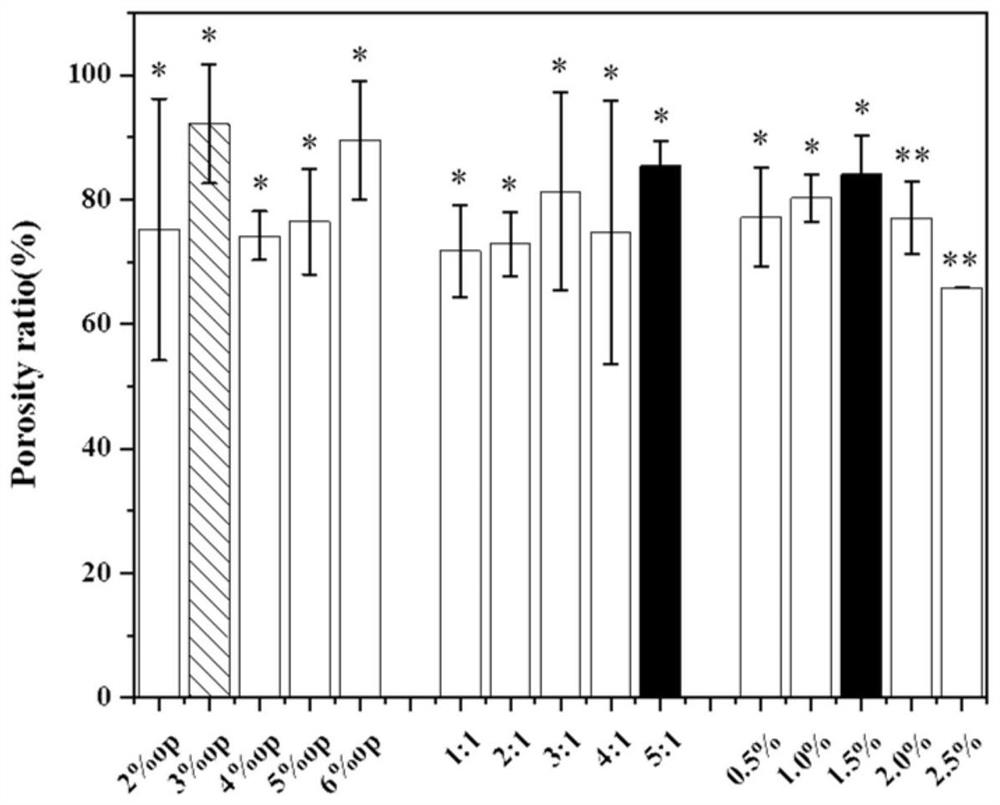
[0035] Measure the liquid absorption and porosity of different amounts of Span 80 (OP) prepared in Example 1, different amounts and different ratios of sodium alginate and cellulose nanocrystal SA / NCC@PL porous microspheres, see the relevant results figure 1 and figure 2 , to obtain two groups of microsphere preparation conditions with better ratio a (4% OP 3:1 1.5%); b (3% OP 5:1 1.5%).
[0036] Determination of the liquid absorption rate of porous microspheres: Weigh the lyophilized SA / NCC@PL porous microspheres as W1, soak them in a centrifuge tube containing deionized water to fully expand. Then use low-speed centrifugation to remove excess liquid, and weigh the wet porous microspheres, denoted as W2. Calculate the liquid absorption rate according to the formula:
[0037]
[0038] Determination of the porosity of the porous microspheres: Soak a certain weight of freeze-dried SA / NCC@PL porous microspheres in 4 mL of absolute ethanol, and oscillate ultrasonically for 1...
Embodiment 3
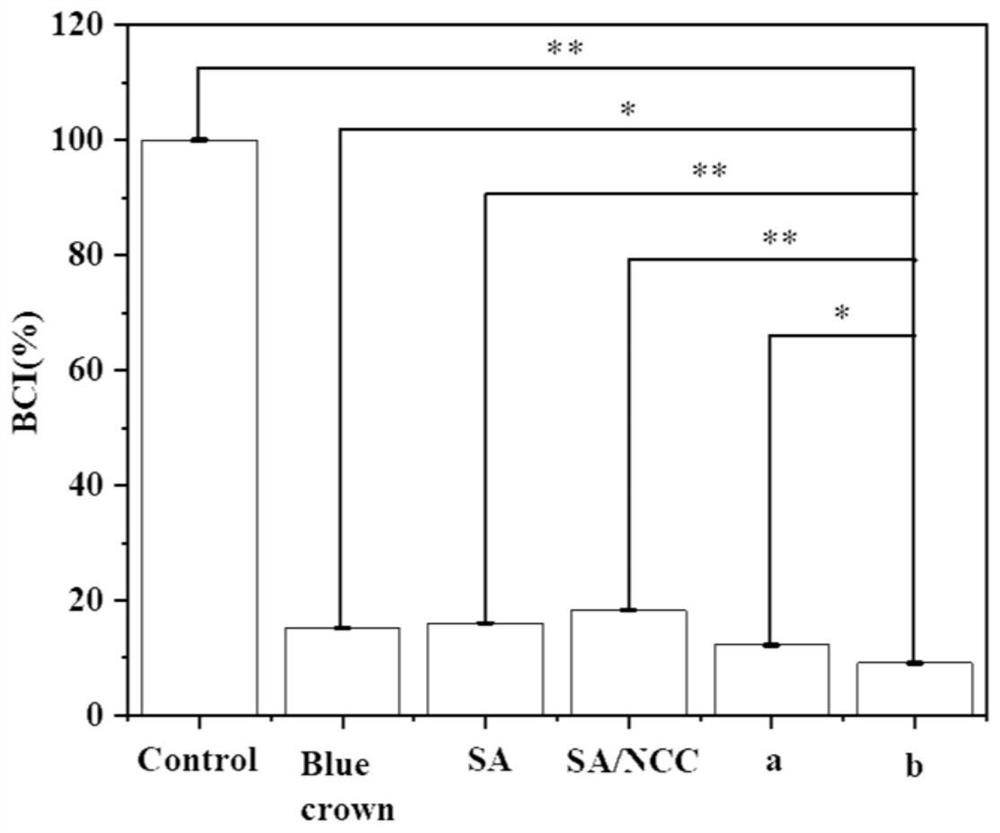
[0041] Comparing the coagulation index (BCI%) of different materials with the negative control group and the commercially available Languan brand chitosan hemostatic powder, the coagulation index of the b (3% OP 5:1 1.5%) group was finally obtained, and the coagulation effect was the best. Related results see image 3 .
[0042] Determination of coagulation index: 10 mg SA / NCC@PL porous microspheres were placed at the bottom of a glass bottle, and preheated at 37°C for 10 minutes. Then add 200ul of fresh anticoagulated rabbit blood and mix well, add 20ul of CaCl 2 The solution was incubated at 37°C for 10 min with mixing. Add 5ml of deionized water to the glass flask and vibrate (50rpm) for 2min to clean uncoagulated red blood cells. Measure the absorbance of the resulting solution at 540 nm with a UV spectrophotometer. The absorbance of 200ul uncoagulated whole blood in 5ml deionized water was used as a negative control. The BCI is calculated according to the following f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com