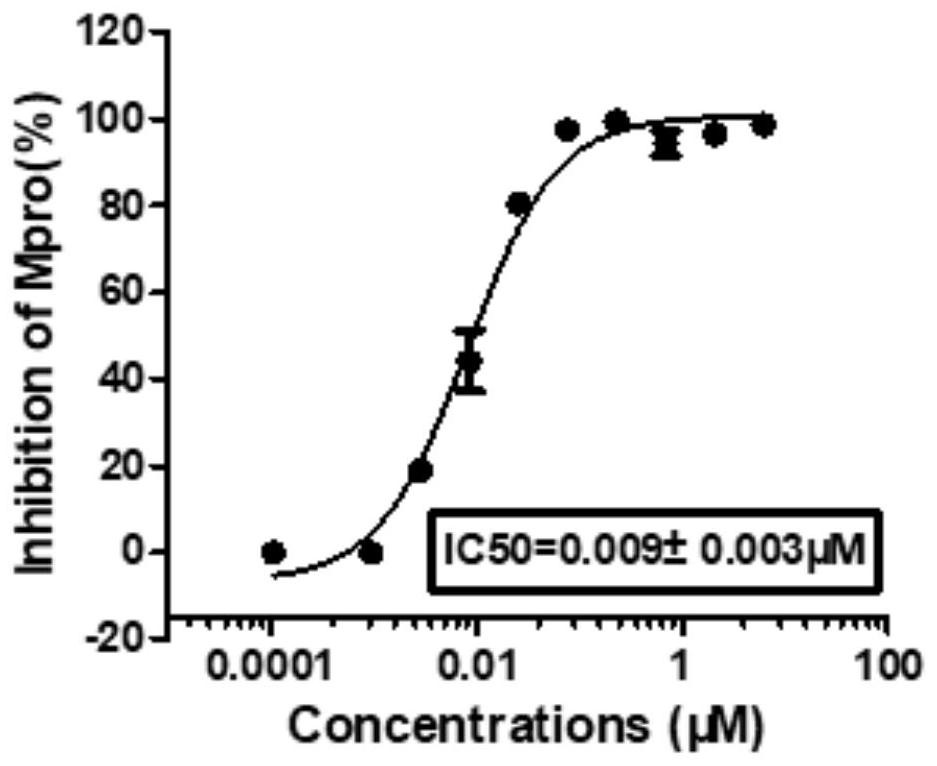
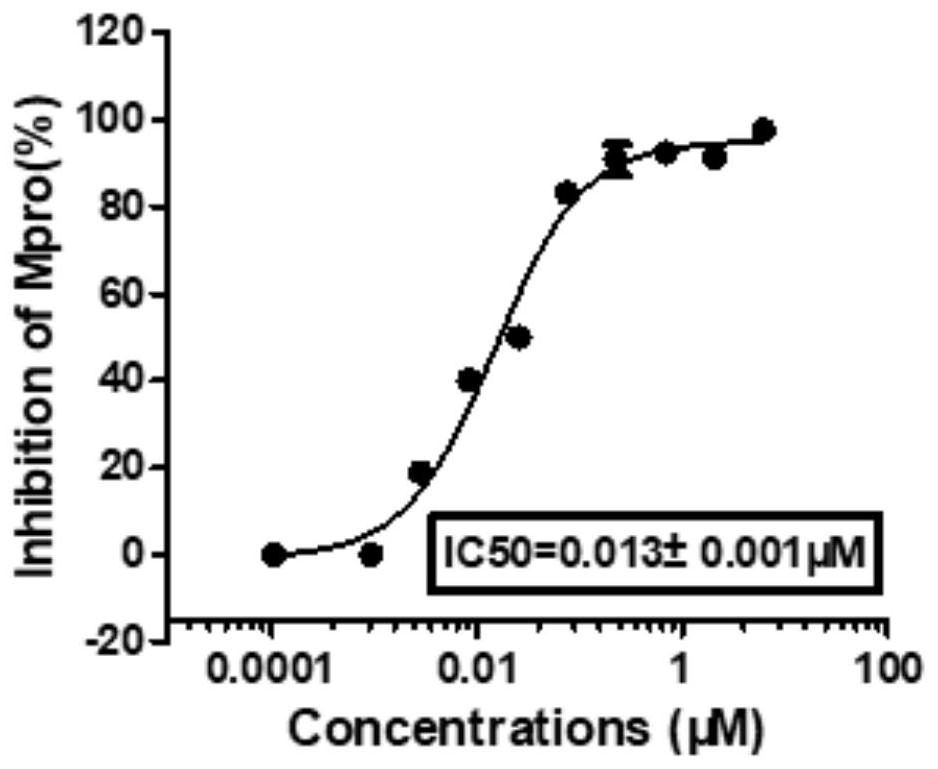
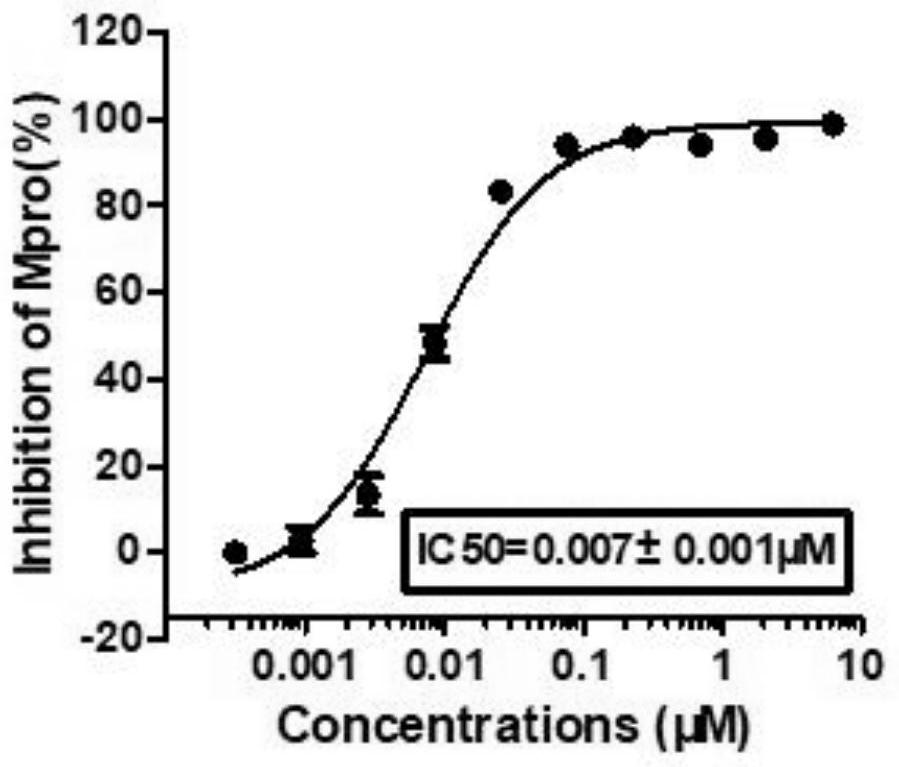
Novel coronavirus main protease inhibitor and preparation method and application thereof
An unsaturated and selected technology, used in antiviral agents, medical preparations containing active ingredients, pharmaceutical formulations, etc., can solve the problems of limited efficacy, toxic and side effects, and achieve pathological damage improvement, reduction in quantity, and good pharmacokinetics. Effects of Dynamic Properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Embodiment 1: the preparation of compound 1
[0081]
[0082] Compound 1 of the present invention is obtained according to the above-mentioned preparation route, and the reaction conditions of each step in the route are as follows:
[0083] i, a, dimethyl 2-fluoromalonate, benzyl alcohol, toluene, p-toluenesulfonic acid, 110°C; b, isopropanol, n-hexane, -10°C;
[0084] ii. Isopropanol, sodium hydroxide, water, 45°C;
[0085] iii. Anhydrous tetrahydrofuran, isopropylmagnesium chloride tetrahydrofuran solution, Ar, 0°C;
[0086] iv. Anhydrous tetrahydrofuran, N,N'-carbonyldiimidazole, Ar, 0°C;
[0087] v, ethyl acetate, 10% palladium carbon, hydrogen, room temperature;
[0088] vi, dichloromethane, dioxane hydrochloride solution;
[0089] vii. Dichloromethane, N,N,N',N'-tetramethyl-O-(7-azabenzotriazol-1-yl)urea hexafluorophosphate, N,N-diisopropyl ethyl Amine, -20°C;
[0090] viii, dichloromethane, trifluoroacetic acid;
[0091] ix, dichloromethane, N,N,N',N'-t...
Embodiment 2
[0111] Embodiment 2: the preparation of compound 3
[0112]
[0113] Compound 3 of the present invention is obtained according to the above-mentioned preparation route, and the reaction conditions of each step in the route are as follows:
[0114] i. Boc-L-dimethyl glutamate, LiHMDS tetrahydrofuran solution, argon, anhydrous tetrahydrofuran, -78°C;
[0115] ii. (2S,4R)-dimethyl 2-(tert-butoxycarbonylamino)-4-(cyanomethyl) glutarate, anhydrous methanol, cobalt chloride hexahydrate, sodium borohydride;
[0116] iii. (S)-methyl 2-(tert-butoxycarbonylamino)-3-((S)-2-carbonylpyrrolidin-3-yl)propionate, lithium hydroxide monohydrate, tetrahydrofuran, 0 ℃;
[0117] iv. Anhydrous tetrahydrofuran, Ar, N,N'-carbonyldiimidazole, 0°C;
[0118] v, ethyl acetate, 10% palladium carbon, hydrogen, room temperature;
[0119] vi, dichloromethane, dioxane hydrochloride solution;
[0120] vii. Dichloromethane, N,N,N',N'-tetramethyl-O-(7-azabenzotriazol-1-yl)urea hexafluorophosphate, N,N-di...
Embodiment 3
[0142] Embodiment 3: preparation compound 9
[0143]
[0144] Compound 9 of the present invention is obtained according to the above-mentioned preparation route, and the reaction conditions of each step in the route are as follows:
[0145] i. 1-hydroxybenzotriazole, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, N,N-diisopropylethylamine, N,N-di Methylformamide, room temperature.
[0146] ii. Sodium hydroxide, methanol, water, 55 degrees
[0147] iii. 2-(7-benzotriazole oxide)-N,N,N',N'-tetramethyluronium hexafluorophosphate, N,N-diisopropylethylamine, N,N-di Methylformamide, 0°C
[0148] iv. Sodium borohydride, methanol, room temperature
[0149] v. Dess Martin oxidant, ultra-dry dichloromethane, room temperature
[0150] The following are the specific synthesis steps:
[0151] Intermediate 25: Methyl(1R,2S,5S)-6,6-Dimethyl 3-(quinoline-2-carbonyl)-3-azabicyclo[3.1.0]hexane-2-carboxylic acid preparation
[0152] Starting material 23 (quinoline 2-carb...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com