Method for synthesizing fisetin
A technology of fisetin and solution, which is applied in the field of drug synthesis, can solve the problems of unsafe reagents, few documents, lengthy reaction procedures, etc., and achieve the effect of reducing synthesis steps, reducing production costs, and shortening synthesis time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
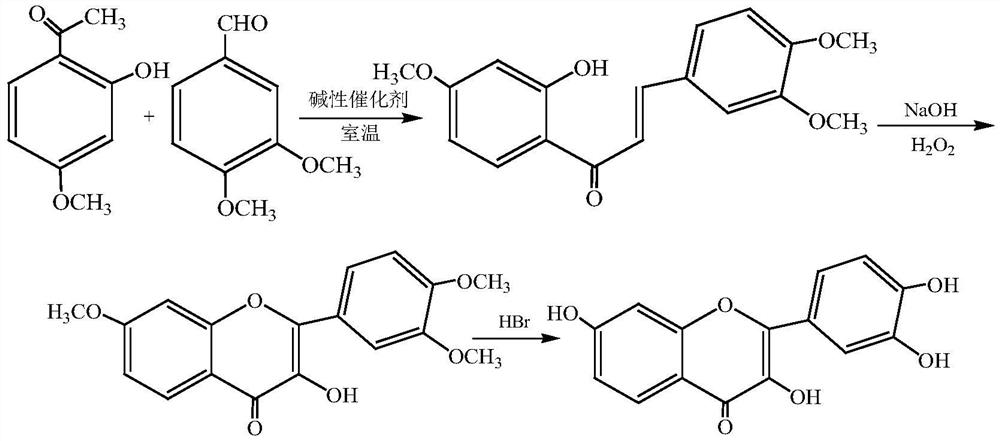
[0028] Step (1).Condensation reaction of paeonol and veratraldehyde
[0029] Weigh 4kg of paeonol and dissolve it in the reaction tank with 81.9L of absolute ethanol; then weigh 4kg of veratraldehyde and dissolve it with absolute ethanol to form a saturated solution; add sodium hydroxide to the ethanol solution of paeonol at 5°C 1.15kg, stir evenly and continue to stir, add the saturated ethanol solution of veratraldehyde into the reaction tank at a constant speed within 0.5h, the reaction solution gradually turns into a light yellow turbid liquid, and then turns into orange yellow, stir and react at 5°C for 11h Afterwards, the reaction solution became more turbid and brown, and the reaction solution was checked by thin-layer chromatography (TLC method). When the raw material point basically disappeared, it was stirred for another 2 h to complete the reaction.
[0030] Step (2). Cyclization reaction of condensation product
[0031] Add 409.6L of 95% ethanol solution with a ma...
Embodiment 2
[0035] Weigh 6kg of paeonol and dissolve it in the reaction tank with 137.3L of absolute ethanol; then weigh 6kg of veratraldehyde and dissolve it with absolute ethanol to form a saturated solution; add sodium hydroxide to the ethanol solution of paeonol at 15°C 1.95kg, start the agitator to stir evenly and continue to stir, add the saturated ethanol solution of veratraldehyde into the reaction tank at a uniform speed within 0.5h, the reaction solution gradually turns into a light yellow turbid solution, and then turns into orange yellow, at 15°C After stirring and reacting for 10 h, the reaction solution became more turbid and brown. The reaction solution was tested by TLC, and then stirred for 1.5 h to complete the reaction. Add 600L of 95% ethanol solution of 15% sodium hydroxide in the reaction tank at one time again, then add 9.4L of 30% hydrogen peroxide solution at a constant speed in the reaction tank within 0.5h, keep stirring for 8h, and then Add 5.0L of 30% hydrogen...
Embodiment 3
[0037] Weigh 9 kg of paeonol and dissolve it in a reaction tank with 195.2 L of absolute ethanol; then weigh 9 kg of veratraldehyde and dissolve it with absolute ethanol to form a saturated solution. Add 2.82kg of sodium hydroxide to the ethanol solution of paeonol at 26°C, start the agitator and stir evenly and continue to stir, add the saturated ethanol solution of veratraldehyde into the reaction tank at a uniform speed within 0.5h, and the reaction solution gradually becomes pale The yellow turbid liquid then turned orange yellow again. After stirring and reacting at 26°C for 10 h, the reaction liquid became more turbid and brown. The reaction liquid was tested by TLC, and then stirred for 1 h to complete the reaction. Add 911L of 95% ethanol solution of 15% sodium hydroxide in the reaction tank at one time again, then add 13L of 30% hydrogen peroxide solution at a constant speed in the reaction tank within 0.5h, keep stirring for 7h, and then Add 7.6L of 30% hydrogen pero...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com