Synthesis method and application of Fe (II)-based specific MRI contrast agent
A synthesis method and contrast agent technology, applied in chemical instruments and methods, analysis by nuclear magnetic resonance, instruments, etc., can solve the problems of indistinguishability and inaccurate acquisition of iron removal therapy information
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
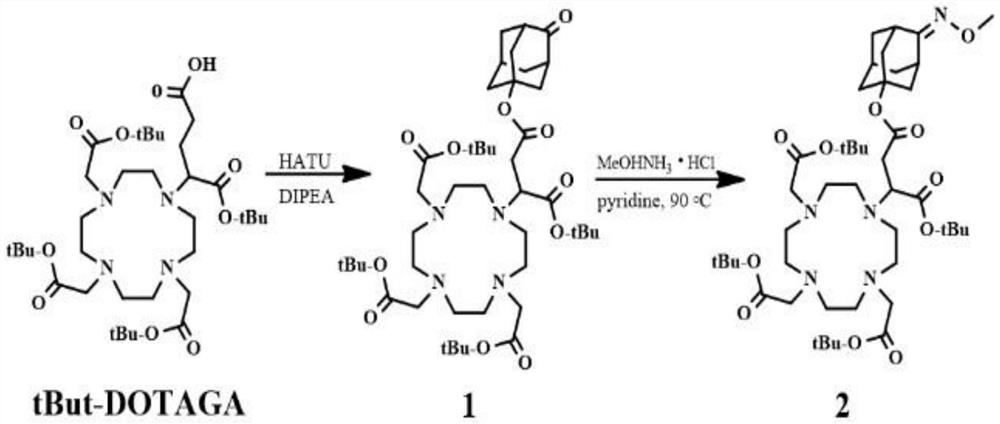
[0028] Embodiment 1: The synthesis method and application thereof based on Fe(II) specific MRI contrast agent provided in this embodiment, specifically include the following steps:
[0029] 1. Synthesis of the first compound: at room temperature, 30mL of DMF containing 6mmol tBut-DOTAGA was added dropwise with 6.2mmol of DIPEA solution, and after 30min, 5mL of HATU (2-(7-azabenzotributazone) dissolved in 6.5mmol was added dropwise azole)-N,N,N',N'-tetramethyluronium hexafluorophosphate) DMF solution; after stirring for 30min, add 6mmol of 5-hydroxyl-2-adamantanone, after stirring for 16 hours, concentrate the resulting solution to dryness and with CH 2 Cl 2 and saturated Na 2 CO 3 aqueous solution extraction, after separation, the organic phase was washed again with saturated aqueous solution, followed by Na 2 CO 3 Washed with brine, washed with Na 2 SO 4 After drying, the first compound is obtained by separation through a chromatographic column.
[0030] 2. Synthesis ...
Embodiment 2
[0034] Embodiment 2: The synthesis method and application thereof based on Fe(II) specific MRI contrast agent provided in this embodiment are basically the same as in Example 1, except that:
[0035] 1. During the synthesis of the first compound: 25 mL of DMF containing 5 mmol tBut-DOTAGA was added dropwise with 5.2 mmol of DIPEA solution, and after 25 min, 3 mL of HATU (2-(7-azabenzotriazepine) dissolved with 6.2 mmol was added dropwise. azole)-N,N,N',N'-tetramethyluronium hexafluorophosphate) DMF solution; after stirring for 35min, add 5mmol of 5-hydroxy-2-adamantanone, and stir for 18 hours.
[0036] 2. During the synthesis of the second compound: 5 mmol of the first compound was dissolved in 10 mL of methanol solution, and 10 mmol of pyridine solution and 6 mmol of methoxyammonium hydrochloride were added dropwise; heated to 80°C in an oil bath and kept for 5 hours.
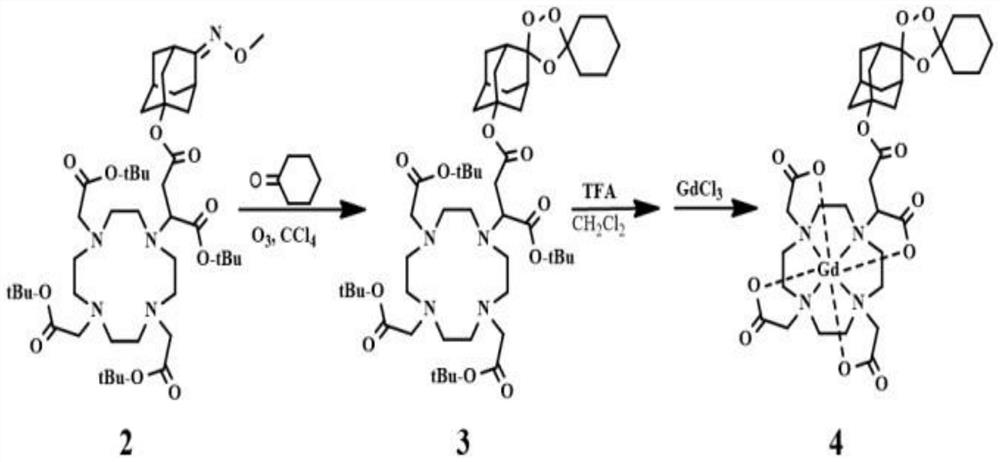
[0037] 3. During the synthesis of the third compound Fer-DOTA: 4mmol carbon tetrachloride solution of the ...
Embodiment 3
[0039] Embodiment 3: The synthesis method and application thereof based on Fe(II) specific MRI contrast agent provided in this embodiment are basically the same as in Example 1, except that:
[0040] 1. During the synthesis of the first compound: 35 mL of DMF containing 6.8 mmol tBut-DOTAGA was added dropwise with 7 mmol of DIPEA solution, and after 35 min, 7 mL of HATU (2-(7-azabenzotriazole) dissolved with 7 mmol was added dropwise. )-N,N,N',N'-tetramethylurea hexafluorophosphate) DMF solution; after stirring for 35min, add 6mmol of 5-hydroxyl-2-adamantanone, and stir for 18 hours.
[0041] 2. During the synthesis of the second compound: 5.5 mmol of the first compound was dissolved in 25 mL of methanol solution, and 13 mmol of pyridine solution and 6.8 mmol of methoxyammonium hydrochloride were added dropwise; heated to 100°C in an oil bath and kept for 5 hours.
[0042] 3. During the synthesis of the third compound Fer-DOTA: 3mmol carbon tetrachloride solution of the second...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com