Method for producing L-ornithine by immobilized enzyme method
A technology for immobilizing enzymes and ornithine, applied in the direction of microorganism-based methods, biochemical equipment and methods, immobilized on or in inorganic carriers, etc., can solve the unfavorable industrialized continuous production of free cell transformation and reduce arginine Enzyme and substrate contact area, separation and purification of unfavorable products and other issues, to achieve the effect of improving catalytic efficiency, improving conversion efficiency, and optimizing composition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: Construction of human recombinant arginase I producing bacteria
[0041] 1. Plasmid transformation:
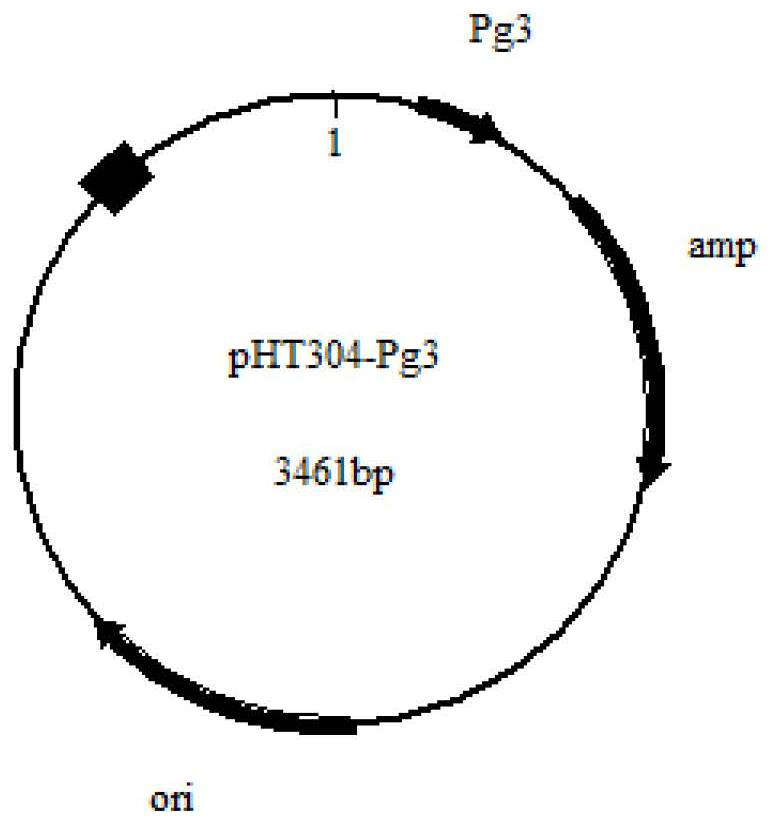
[0042] The pHT304 plasmid (purchased from Youbao Biology) was digested with NdeI and HincII, and then the Pg3 promoter (sequence shown in SEQ ID NO.1) was integrated into the pHT304 plasmid after double digestion to construct the plasmid pHT304-Pg3( figure 1 ).
[0043] The constructed plasmid pHT304-Pg3 is resistant to single ampicillin, has a lactose operon, and its nucleotide sequence is shown in SEQ ID NO.2.
[0044] The purpose of transforming the pHT304 plasmid is: first, to reduce the length of the vector and improve its expression stability in Bacillus subtilis; second, to integrate the Pg3 promoter into the pHT304 plasmid, and the Pg3 promoter only starts after adding IPTG Therefore, after integrating the Pg3 promoter, the strain grows fast in the early stage, and the time required to enter the stable phase is short, thereby shortening the expr...
Embodiment 2
[0063] Embodiment 2: the fermentative production of human recombinant arginase I
[0064] (1) Strain activation:
[0065] Streak-inoculate the human recombinant arginase I producing bacteria constructed in Example 1 onto an LB plate containing 100 μg / ml ampicillin, and culture at 33° C. for 24 hours.
[0066] (2) First-level species cultivation:
[0067] One inoculation loop of bacteria was drawn from the plate and transferred to primary seed medium (LB liquid medium supplemented with 50 ppm ampicillin), and cultured at 33° C., pH 7.0, and rotational speed 200 rpm for 18 hours.
[0068] (3) Secondary species cultivation:
[0069] With 1% (volume fraction) inoculation amount, inoculate primary seed liquid in secondary seed medium (LB liquid medium that adds 50ppm ampicillin), 33 ℃, dissolved oxygen (DO) 20-40%, cultivate to Dilute 100 times OD 600nm Value 0.5.
[0070] (4) Fermentation culture:
[0071] The secondary seed liquid of L-ornithine producing bacteria is inocul...
Embodiment 3
[0075] Embodiment 3: Production of L-ornithine by immobilized enzyme method
[0076] (1) Arginase immobilization treatment:
[0077] Adjust the pH value of the fermented broth after the induction culture in Example 2 to 7.2, and break it through a homogenizer. The mixed and homogenized cells after crushing are passed through a ceramic membrane, and the filtrate is collected; the pore diameter of the ceramic membrane is 100 nm, and the pressure is 0.5 MPa.
[0078] Dilute the filtrate to arginase concentration of 10g / L, add PBS to make the concentration of PBS 50mM, pass through nickel column, flow rate 3m 3 / h.
[0079] Nickel column affinity chromatography conditions: 50mM PBS was used as the binding buffer, and the filtrate was hung on the column.
[0080] (2) L-arginine conversion:
[0081] Substrate solution (containing L-arginine 50g / L, pyridoxal phosphate 1mg / L, dimethyl sulfoxide 0.1mg / L) in 3m 3 The flow rate of / h flows through the nickel column after the column ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com