Doxorubicin prodrug for combined use of tumor penetration enhanced photo-thermal and chemotherapy and preparation method of doxorubicin prodrug
A technology for enhancing penetration and doxorubicin is applied in the field of doxorubicin prodrug and its preparation, which can solve the problems of reducing the efficacy of chemotherapeutic drugs, and achieve the effects of avoiding early release and improving drug utilization rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
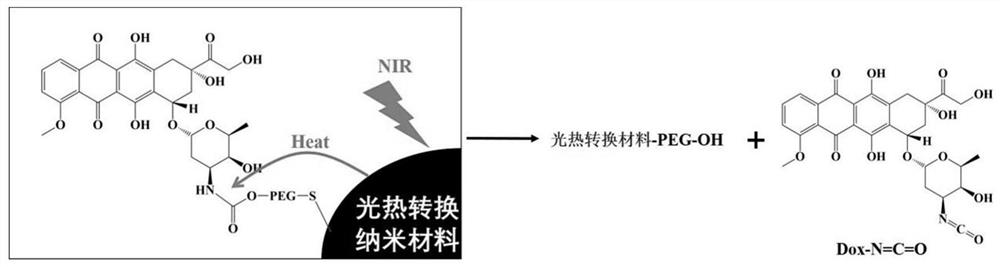
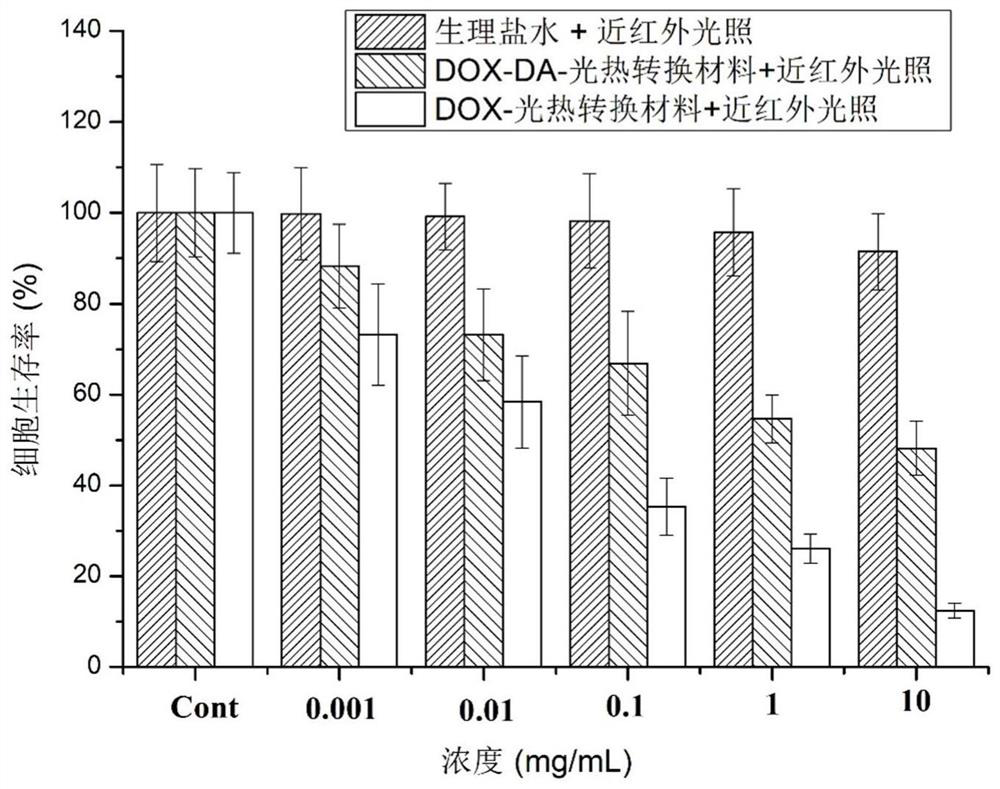
[0037]1) Preparation of DOX-photothermal conversion nanoparticles: 0.1 mmol DOX hydrochloride and 0.1 mmol NPC-PEG-SH (molecular weight 1 KDa) were dissolved in 3 mL DMF, and the reaction mixture was stirred at room temperature under nitrogen atmosphere for 18 hours. After purification, DOX-NH-COO-PEG-SH was produced. Then connect 20 mg of near-infrared photothermal conversion material copper sulfide to the thiol end to obtain DOX-photothermal conversion nanoparticles;
[0038] 2) Preparation of pHPP nanocarrier: Dissolve 0.1mol PCL-COOH (molecular weight 11.4KDa) in N,N-dimethylformamide (DMF), then add the same molar amount of EDC and NHS, react at room temperature for 2-3 hours to activate the carboxyl group on PCL-COOH, then add 0.3mmol of N 2 h 4 ·H 2 O was reacted overnight. It was then dialyzed against deionized water and lyophilized to obtain pHPNN. PEG-ALD (molecular weight 5KDa) and pHPNN were mixed and dissolved in ethanol in equimolar amounts, and heated to re...
Embodiment 2
[0041] 1) Preparation of DOX-photothermal conversion nanoparticles: 0.1 mmol DOX hydrochloride and 0.09 mmol NPC-PEG-SH (molecular weight 5K Da) were dissolved in 3 mL DMF, and the reaction mixture was stirred at room temperature under nitrogen atmosphere for 18 hours. After purification, DOX-NH-COO-PEG-SH was produced. Then connect 30 mg of near-infrared photothermal conversion material gold nanorods with the thiol end to obtain DOX-photothermal conversion nanoparticles;
[0042] 2) Preparation of pHPP nanocarrier: Dissolve 0.1mol PCL-COOH (molecular weight 10K Da) in N, N-dimethylformamide (DMF), then add the same molar amount of EDC and NHS, and react at room temperature for 2- 3 hours to activate the carboxyl group on PCL-COOH, then add 0.25mmol N 2 h 4 ·H 2 O was reacted overnight. It was then dialyzed against deionized water and lyophilized to obtain pHPNN. PEG-ALD (molecular weight 2K Da) and pHPNN were mixed and dissolved in ethanol in equimolar amounts, and heate...
Embodiment 3
[0045] 1) Preparation of DOX-photothermal conversion nanoparticles: 0.1 mmol DOX hydrochloride and 0.11 mmol NPC-PEG-SH (molecular weight 8 KDa) were dissolved in 3 mL DMF, and the reaction mixture was stirred at room temperature under nitrogen atmosphere for 18 hours. After purification, DOX-NH-COO-PEG-SH was prepared, and 40 mg of near-infrared photothermal conversion material copper sulfide was connected to the thiol end to obtain DOX-photothermal conversion nanoparticles;
[0046] 2) Preparation of pHPP nanocarrier: Dissolve 0.1mmol PCL-COOH (molecular weight 5K Da) in DMF, then add the same molar amount of EDC and NHS, and react at room temperature for 2 to 3 hours to activate the carboxyl group on PCL-COOH , then add 0.4mmol N 2 h 4 ·H 2 O was reacted overnight. It was then dialyzed against deionized water and lyophilized to obtain pHPNN. PEG-ALD (molecular weight 5K Da) and pHPNN were mixed and dissolved in ethanol at a molar ratio of 1:1.1, and heated to reflux for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com