Preparation method of carboxylic acid compound and metal salt derivative thereof
A technology of compounds and carboxylic acids, which is applied in the field of preparation of carboxylic acid compounds and their metal salt derivatives, can solve problems such as non-industrial synthesis methods, achieve high practical value, broad application prospects, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
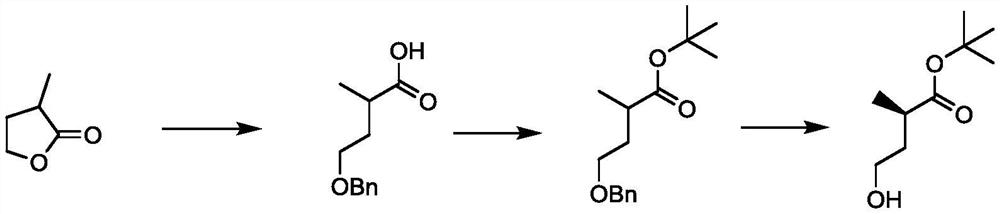
[0052] Due to existing compound 1 and compound 2 The preparation method of the present invention has problems such as cumbersome steps, expensive raw materials or harsh reaction conditions, which leads to high production costs, is not suitable for industrial production, and restricts the development of some medicines. Therefore, the inventor has carried out a large number of experiments, and proposes a new preparation method of carboxylic acid compounds, which uses raw materials from a wide range of sources, and has a simple process and mild reaction conditions.
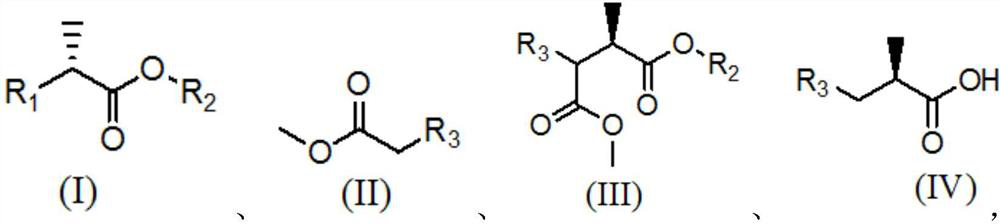
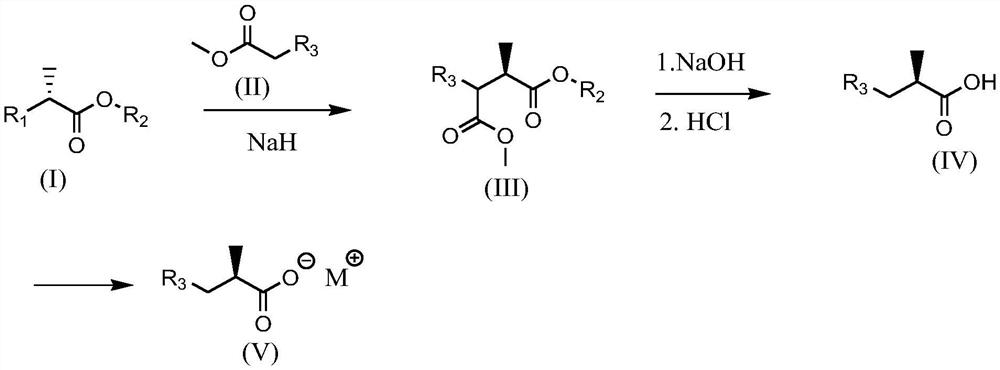
[0053] Reaction equation of the present invention is:
[0054]
[0055] In the above reaction formula, R 1 Is halogen or sulfonic acid group; R 2 for C 1 ~C 6 Alkyl; R 3 is cyano or -COOR 4 , R 4 for C 1 ~C 4 Alkyl group, M is monovalent metal ion such as Na, K, etc., the yield of carboxylic acid compound can reach 70% and above, and the yield of its metal salt derivative can reach 67%.
Embodiment 1
[0058] 1) D-tert-butyl lactate sulfonylation
[0059]
[0060]Add 80ml of dichloromethane, 14.2g (100mmol) of D-tert-butyl lactate, 12.3g (120mmol) of triethylamine, 21.9g (115mmol) of p-toluenesulfonyl chloride into the reaction flask, and react at 30°C-35°C for 5 hours. Add 50 g of water to wash the organic layer, add anhydrous sodium sulfate to the organic layer to dry, filter off the desiccant, concentrate, add 50 ml of tetrahydrofuran to dilute and directly use in the next reaction.
[0061] 2) Preparation of (R)-3-methyl-4-tert-butoxy-4-oxobutanoic acid
[0062]
[0063] Add 80ml of tetrahydrofuran, 14.5 grams (110mmol) of dimethyl malonate, 3.8 grams of 60% sodium hydrogen (110mmol) in the reaction flask, and dropwise add the tetrahydrofuran solution prepared in Example 1 at 35 ° C. After the dropwise addition, 50 ℃ for another 3 hours, concentrated under reduced pressure to remove the solvent, added 50ml of toluene and 60ml of 4N sodium hydroxide solution, react...
Embodiment 2
[0067]
[0068] Add 80ml of tetrahydrofuran, 14.5 grams (110mmol) of dimethyl malonate, 3.8 grams of 60% sodium hydrogen (110mmol) into the reaction flask, and add 16.6 grams (100mmol) of (R)-2-tert-butyl chloropropionate dropwise at 35°C After the addition of the ester, react at 50°C for another 3 hours, concentrate under reduced pressure to remove the solvent, add 50ml of toluene and 60ml of 4N sodium hydroxide solution, react at room temperature for 2 hours, add hydrochloric acid dropwise to neutralize to PH=1-2, and heat to reflux 1 hour, cooled to room temperature, separated the water layer, concentrated the organic layer, added petroleum ether to precipitate a solid, filtered, and dried to obtain 14.1 g of the product, with a purity of 98% and an optical purity of ee% of 97%.
[0069] Product hydrogen spectrum:
[0070] 1 H NMR (400MHz, CDCl 3 ):11.0(s,1H),2.92-2.77(m,2H),2.64-2.53(m,1H),1.38(s,9H),1.19(s,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com