Application of acylated phloroglucinol derivative in preparation of antibacterial agent
The technology of acyl phloroglucinol and phloroglucinol is applied in the field of antibacterial activity of phloroglucinol derivatives, and can solve the problems of poor sensory quality, decreased nutritional value, infiltration of mycotoxins and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
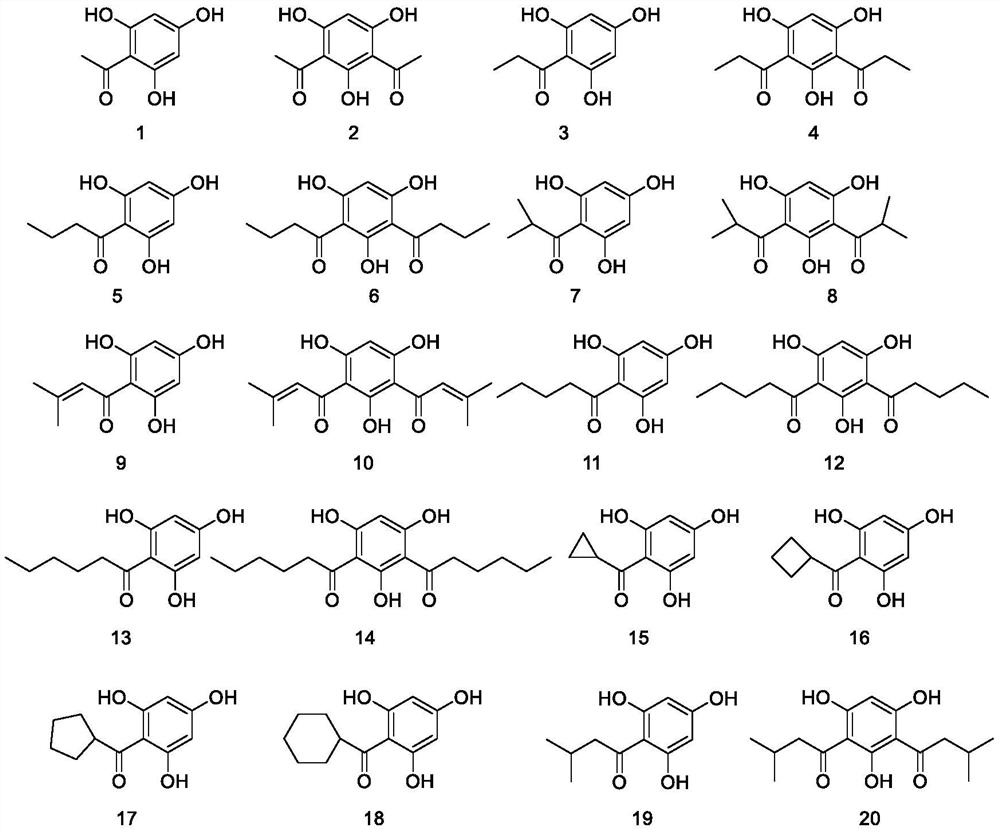
[0016] Embodiment 1: the preparation of phloroglucinol derivative
[0017] Compound 1-20 (refer to European Journal of Medicinal Chemistry 155 (2018) 255e262; J Antibiot (Tokyo) 2019, 72 (5), 253-259; J. Nat. Prod. 2016, 79, 2195-2201) synthesis, The phloroglucinol derivatives 1-20 listed in the claims are determined according to the physical properties and NMR mass spectrometry identification consistent with the literature reports. All compounds were separated by conventional methods such as silica gel column chromatography to obtain pure products for further activity testing.
Embodiment 2
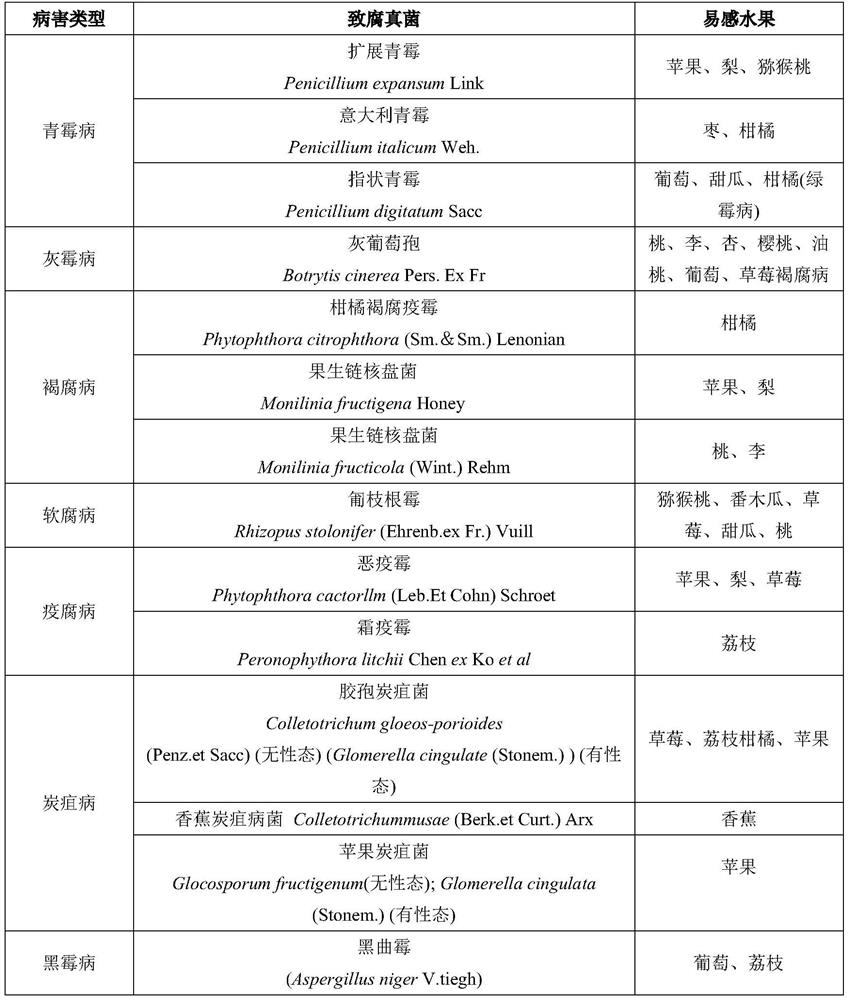
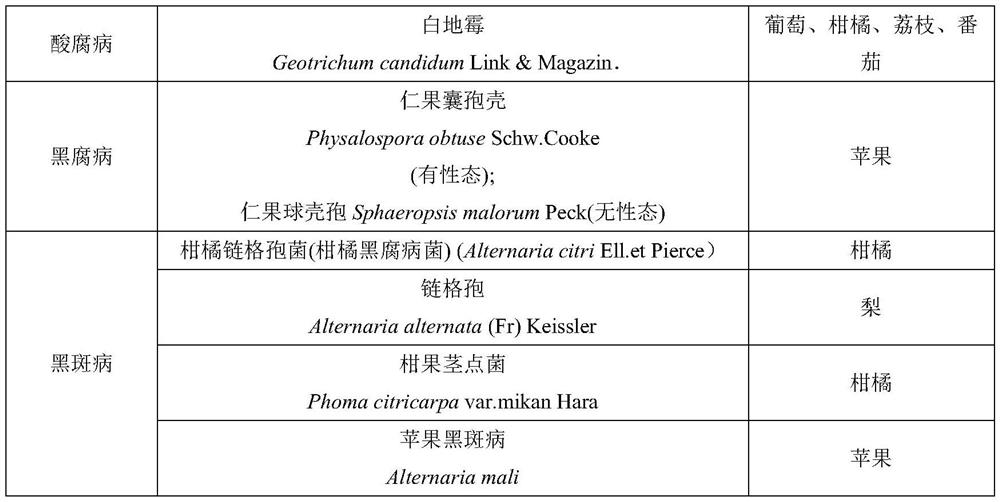
[0018] Example 2: Determination of Indoor Antibacterial Activity of Phloroglucinol Derivatives on Postharvest Fruit Rot-causing Fungi
[0019] The phytopathogenic fungi used in this experiment were strains stored at 4°C in the laboratory. The mycelial growth rate method was used. The medium used for all phytopathogenic fungi in the laboratory was potato agar dextrose medium (PDA for short). PDA medium formula: potato (peeled) 200g, glucose 20g, agar 15g, distilled water 1000mL.
[0020] PDA medium preparation method: wash and peel the potatoes, weigh 200g and cut into small pieces, add water and boil until it can be punctured by a glass rod, filter with four layers of gauze, add distilled water in a beaker To 1000 mL, add 15-20 g of agar according to the needs of the experiment, add 20 g of glucose, stir to dissolve it fully, then divide it into Erlenmeyer flasks, sterilize at 121°C for 20 minutes, and cool down for later use.
[0021] experimental method
[0022] 1) Mycelia...
Embodiment 3
[0034] Embodiment 3: Determination of indoor antibacterial activity of phloroglucinol derivatives to plant pathogenic fungi
[0035] Indoor bacteriostatic activity of table 4 phloroglucinol derivatives to phytopathogenic fungi
[0036]
[0037] As can be seen from the bioassay results in Table 4, the phloroglucinol derivatives involved in the present invention have good antibacterial activity to 5 kinds of plant pathogenic fungi, and when the acyl side chain alkane contains branched chains, the antibacterial activity significantly decreases, and it is a linear alkane Or cycloalkane antibacterial activity is significant.
[0038] Embodiment 3: Determination of indoor antibacterial activity of phloroglucinol derivatives to agricultural bacteria
[0039]The micro-broth dilution method was adopted: the agricultural bacteria were inoculated in 20mL NB medium, and then placed in a constant temperature shaker at 37°C for 12h, with a rotation speed of 150rpm. Take an appropriate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com