Interpenetrating network structure layer and in-situ preparation method and application thereof
A technology of interpenetrating network structure and in-situ preparation, applied in the field of interpenetrating network structure electrolyte interface layer and in-situ preparation, can solve problems such as interface problems, poor air stability, poor interface stability, etc. Mechanical strength, effect of inhibiting the growth of lithium dendrites
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
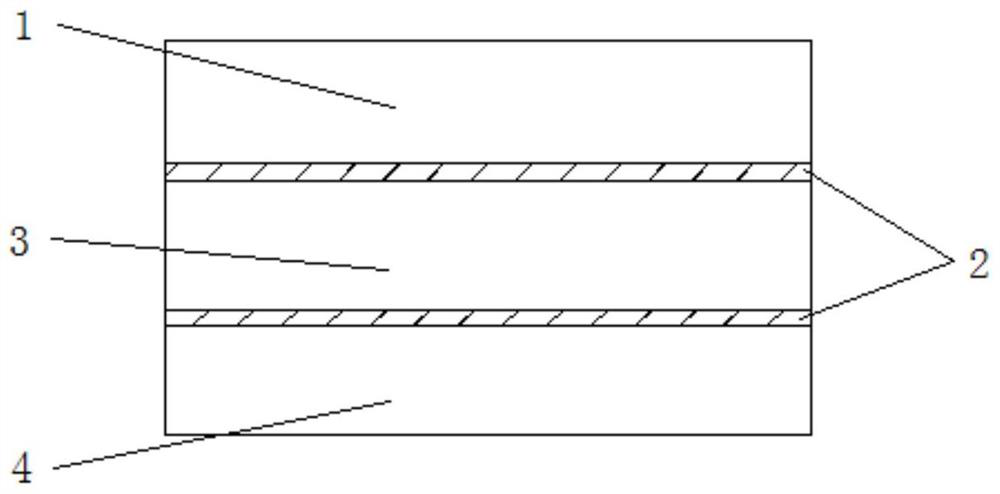
[0041] In this embodiment, a method for in-situ preparation of an interpenetrating network structure electrolyte interface layer includes the following steps:
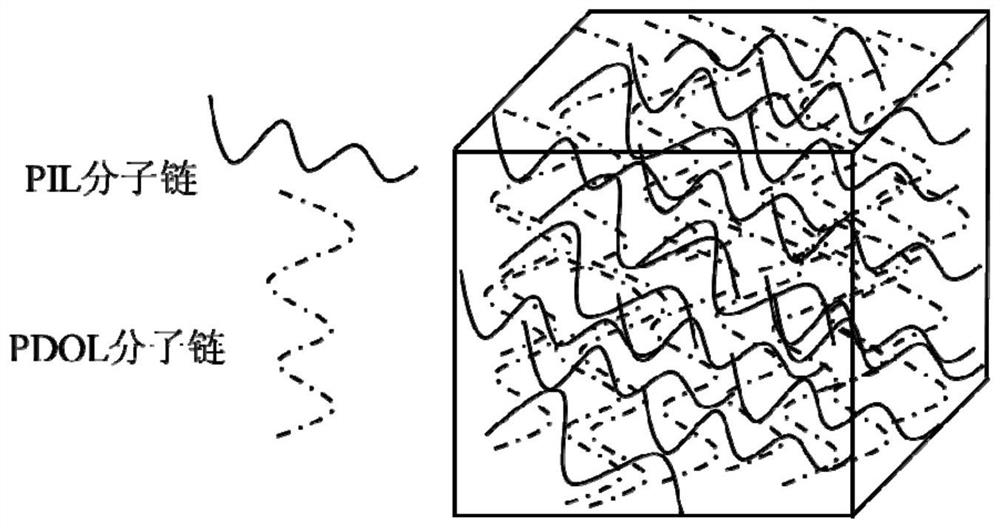
[0042] (1) Preparation of interfacial layer precursor: under the atmosphere of protective gas, first mix 1g of 1-vinyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide salt) (VMImTFSI) and LiTFSI (20mol% ) to mix, then add photoinitiator 2-hydroxyl-2-methylpropiophenone and mix evenly, UV light polymerization to obtain polyionic liquid (PIL), then 2mL 1,3 dioxolane (DOL) and LiPF 6 The initiator is mixed evenly and added to the PIL to make it absorbed to obtain the interface layer precursor gel;
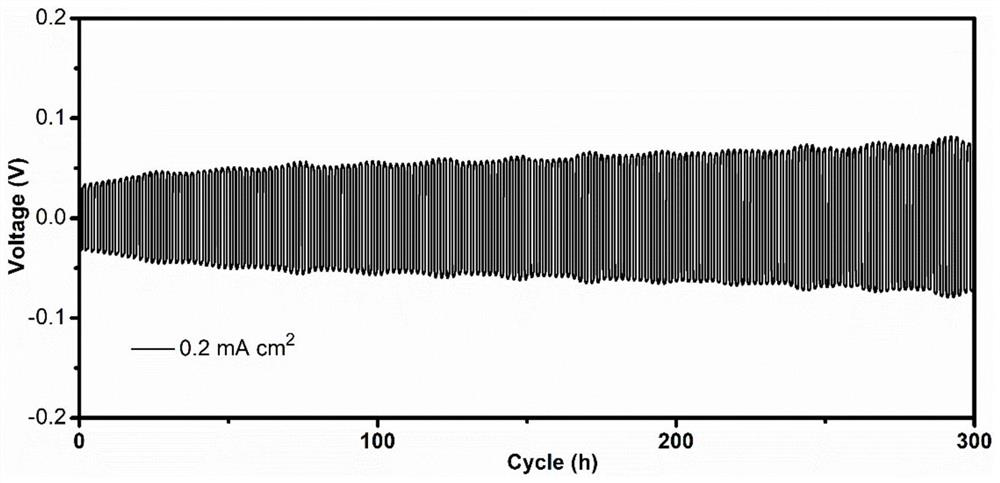
[0043] (2) 200mg of inorganic solid electrolyte Li 10 GeP 2 S 12 Press into a ceramic sheet with a diameter of 12 mm, place the above precursor solution on the surface of the inorganic electrolyte sheet, let it stand at room temperature, pass LiPF 6 Initiated cationic polymerization results in an electrolyte interfa...
Embodiment 2
[0049] The difference between this embodiment and embodiment 1 is:
[0050] (1) Preparation of interfacial layer precursor: under the atmosphere of protective gas, first mix 1g of 1-vinyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide salt) (VMImTFSI) and LiTFSI (60mol% ) to mix, then add photoinitiator 2-hydroxyl-2-methylpropiophenone and mix evenly, UV light polymerization to obtain polyionic liquid (PIL), then 2mL 1,3 dioxolane (DOL) and LiPF 6 The initiator is mixed evenly and added to the PIL to make it absorbed to obtain the interface layer precursor gel;
[0051] (2) 200mg of inorganic solid electrolyte Li 10 GeP 2 S 12 Press into a ceramic sheet with a diameter of 12mm, place the above-mentioned precursor on the surface of the inorganic electrolyte sheet, let it stand at room temperature, pass LiPF 6 Initiated ring-opening polymerization yields an electrolyte interfacial layer with an interpenetrating network structure.
[0052] (3) Assembling the inorgani...
Embodiment 3
[0054] The difference between this embodiment and embodiment 1 is:
[0055] (1) Preparation of interfacial layer precursor: under the atmosphere of protective gas, firstly mix 2mL 1,3 dioxolane (DOL) and LiPF 6 Mix well, stand for 6h, ring-opening polymerization to obtain polyether molecular network polymer, then 1g 1-vinyl-3-butylimidazole bis(trifluoromethanesulfonyl)imide salt) (VBImTFSI) and LiTFSI (80mol %), photoinitiator 2-hydroxyl-2-methyl propiophenone mix evenly, join in the above-mentioned molecular network polymer, make it absorb, obtain interface layer precursor gel;
[0056] (2) 200mg of inorganic solid electrolyte Li 10 GeP 2 S 12 Press into a ceramic sheet with a diameter of 12 mm, place the above precursor on the surface of the inorganic electrolyte sheet, and irradiate with ultraviolet light to obtain an electrolyte interface layer with an interpenetrating network structure.
[0057] (3) Assembling the inorganic solid-state electrolyte with the interfacia...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com