Stable mutants of quorum quenching lactonase and their use in treatment of pathogens
A lactonase, mutant-based technology for the management of fire blight and other plant diseases that addresses unmet issues of effective reagents and management, regulatory constraints, and public health restrictions on the long-term prospects for use of antibiotics and other reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0138] Example 1. Recombinant expression, purification and biochemical characterization of enzymes utilizing c6-oxo-HSL
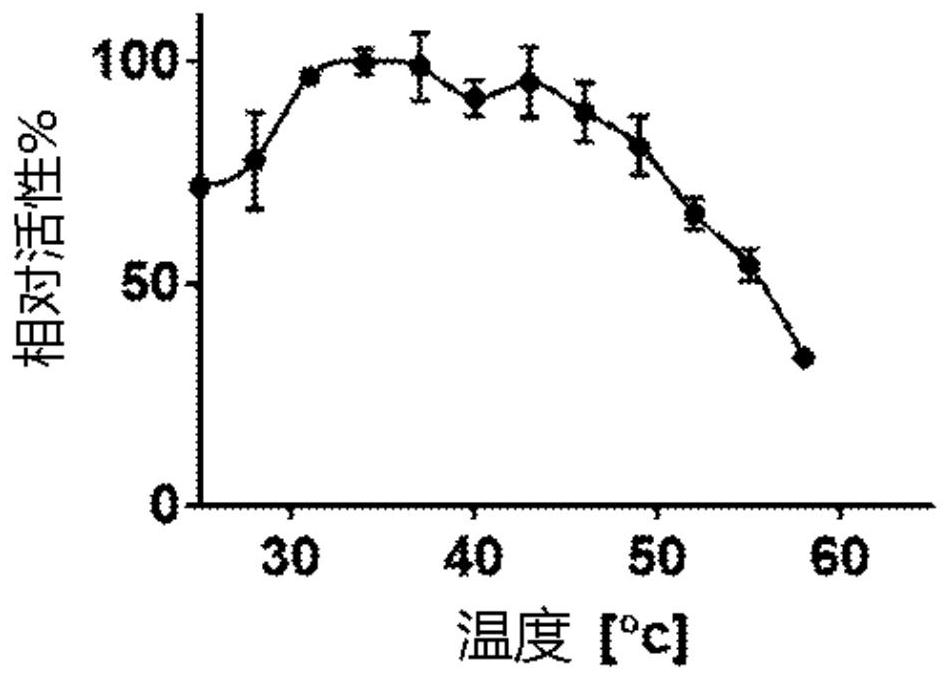
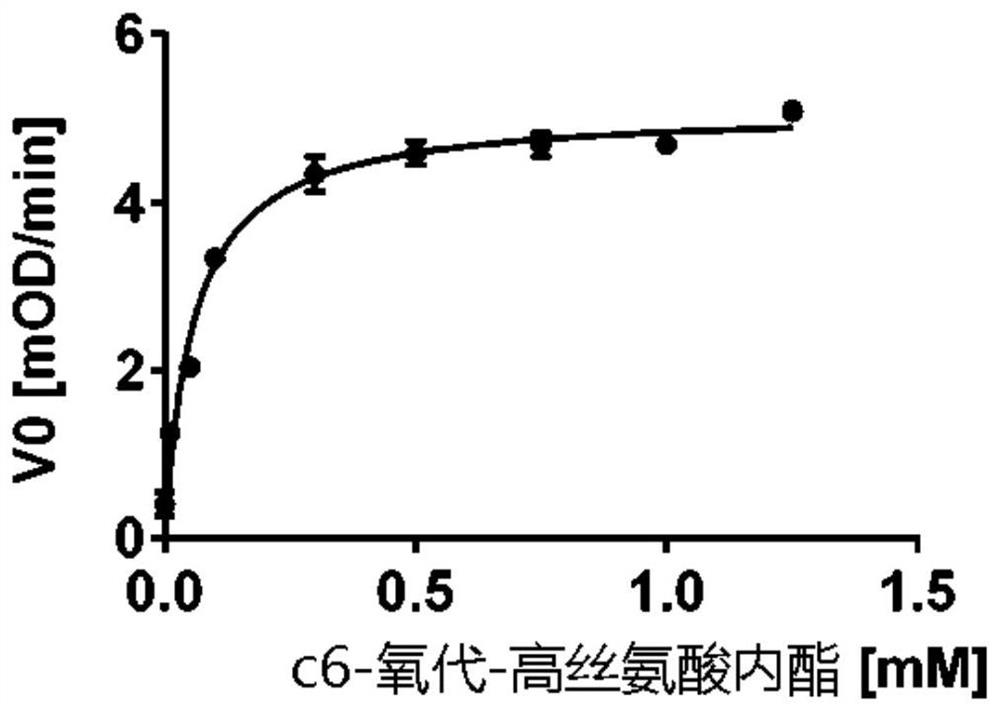
[0139] The genes encoding PPH and its evolutionary variants from Mycobacterium tuberculosis were cloned into the expression vector pMal-c4X and propagated in Escherichia coli-BL21(DE3) as a fusion protein with the high-binding mutant (A313V) maltose-binding protein. Expression (SEQ ID NO: 10). Cells were then lysed and proteins were purified using an amylose column (NEB). After purification, we tested the temperature optima of the enzymes, their thermostability and shelf life with a chromogenic substrate (TBBL, thiobutyrylbutyrolactone). Their association with C6-oxo-HSL (also known as N-hexanoyl-L-homoserine) as a lactone secreted by the plant pathogen Erwinia amylovora was tested using a pH indicator as previously described (7). Lactone, N-[(3S)-tetrahydro-2-oxo-3-furyl]hexanamide, HHL), see Figure 1B and 2A ; PPH exhibits high activity with C6-oxo-H...
Embodiment 2
[0140] Example 2. Construction of random gene libraries and isolation of improved variants with higher activity, thermostability and shelf life
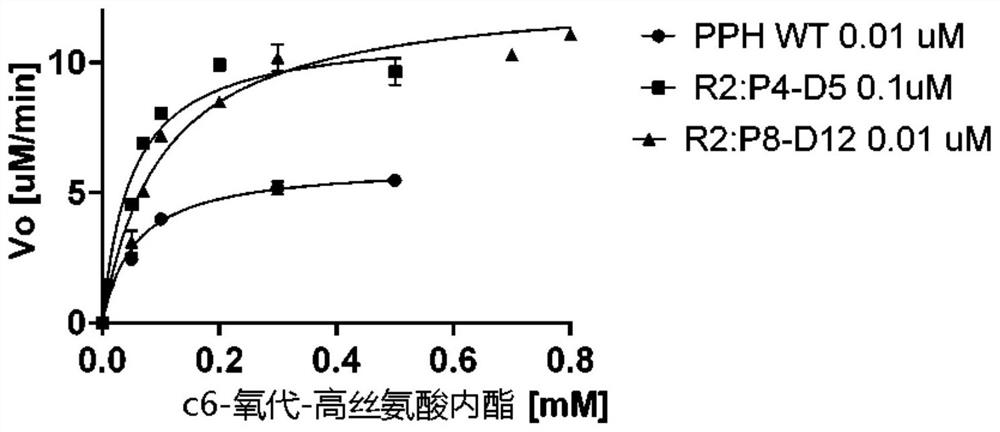
[0141] The gene encoding PPH was used to construct a gene library using the Gene Morph Random Mutagenesis Kit (Agilent). After PCR amplification, the resulting PCR product was used as a template for nested PCR with outer primers, followed by digestion with EcoRI and PstI and ligation into pMAL-c2x, the library plasmid was electroporated into E. coli DH5α, and the estimated library size was 5000 , and isolate plasmid DNA. Single clones of unselected libraries were sequenced and an average of 2-3 point mutations per gene were identified. Use rounds of random mutations (3-4*10 5 library size of variants) and screening (600 variants per round) for increased thermostability, we isolated two variants with unique sequences, see Figure 2: variants with the following mutations: variant PPH_R2 : G58V [G59V; SEQ ID NO: 11] in P4-D5 and varia...
Embodiment 3
[0143] Example 3. Wild-type QQ PPH lactonase inhibits exopolysaccharide formation in culture
[0144] Levan production was observed spectroscopically at 400 nm after supplementation with 500 mM sucrose as a measure of bacterial virulence manifested in the formation of the exopolysaccharide (EPS) matrix according to a previously described protocol (Molina et al., 2005). .
[0145] When the purified wild-type PPH protein was applied to the cell culture of Erwinia amylovora, a 30% decrease in EPS production was observed ( image 3 ). The G59V and H172Y mutants are expected to be effective at least in reducing EPS production.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal stability | aaaaa | aaaaa |
| thermal stability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com