Histidine-labeled fluorescent probe as well as preparation method and application thereof
A technology of fluorescent probes and fluorescent compounds, which is applied in the fields of chemistry and biology, can solve the problems of slow protein labeling, complex synthesis methods, and inapplicability, and achieve the effects of strong specificity, high sensitivity, and reduced interference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
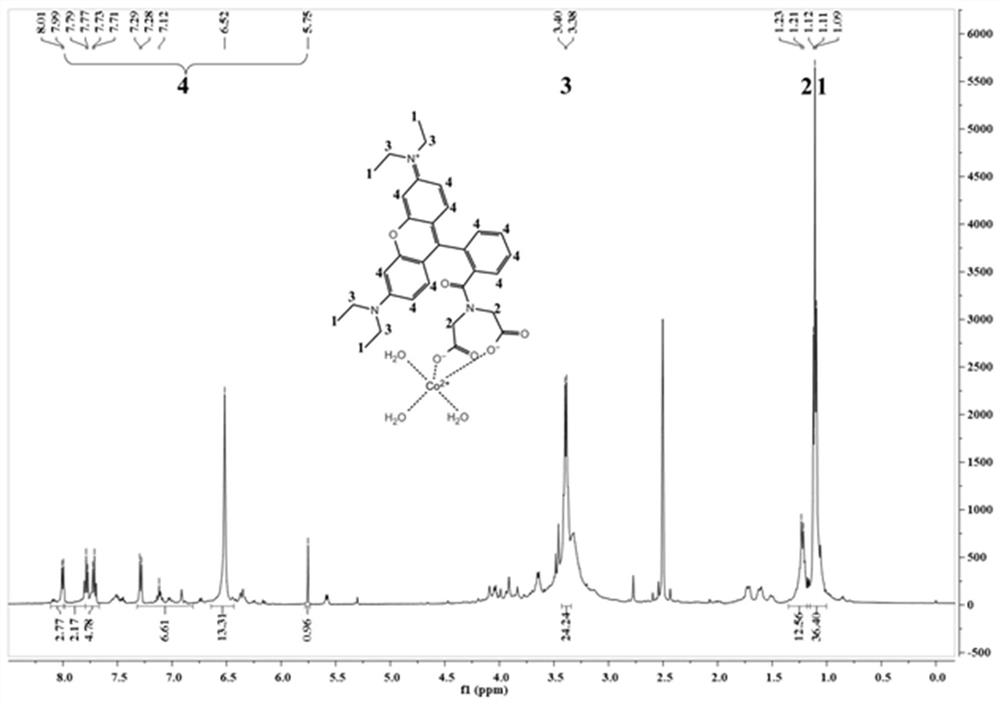
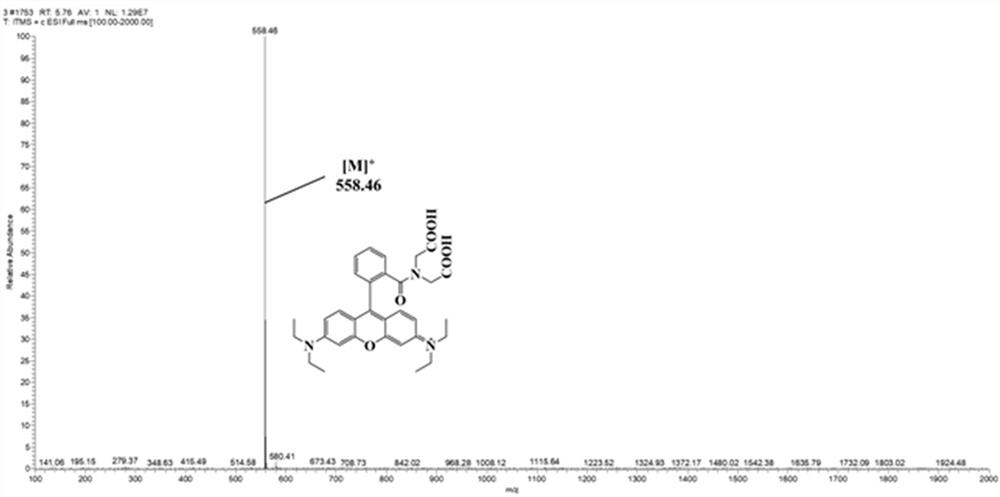
[0032] Embodiment 1: the chemical synthesis of fluorescent probe Rho-IDA
[0033] 1) In a 50 mL reaction flask equipped with a magnetic stirrer, add 0.1 mmol of rhodamine B and 10 mL of dichloromethane, and fully dissolve.
[0034] 2) Slowly add 0.15 mmol N-hydroxysuccinimide and 0.15 mmol N, N'-dicyclohexylcarbodiimide, react at room temperature overnight, and filter to remove the precipitate.
[0035] 3) Slowly add 0.2 mmol of triethylamine and mix well.
[0036] 4) Add 0.15 mmol of iminodiacetic acid and react overnight at room temperature.
[0037] 5) Evaporate the solvent under reduced pressure, dissolve the residue with 30 mL of dichloromethane, continue to add 30 mL of saturated saline for extraction, and separate the dichloromethane layer.
[0038] 6) Filter to remove impurities, use silica gel column chromatography, the eluent is petroleum ether:ethyl acetate=5:1, and obtain rose bengal powder with a total yield of 64%.
[0039] The synthetic product obtained above...
Embodiment 2
[0040] Embodiment 2: Fluorescent performance measurement of fluorescent probe Rho-IDA-CoII
[0041] 1) The same molar concentration of Rho-IDA and CoCl 2 Equal volumes were mixed to obtain the fluorescent probe Rho-IDA-CoII;
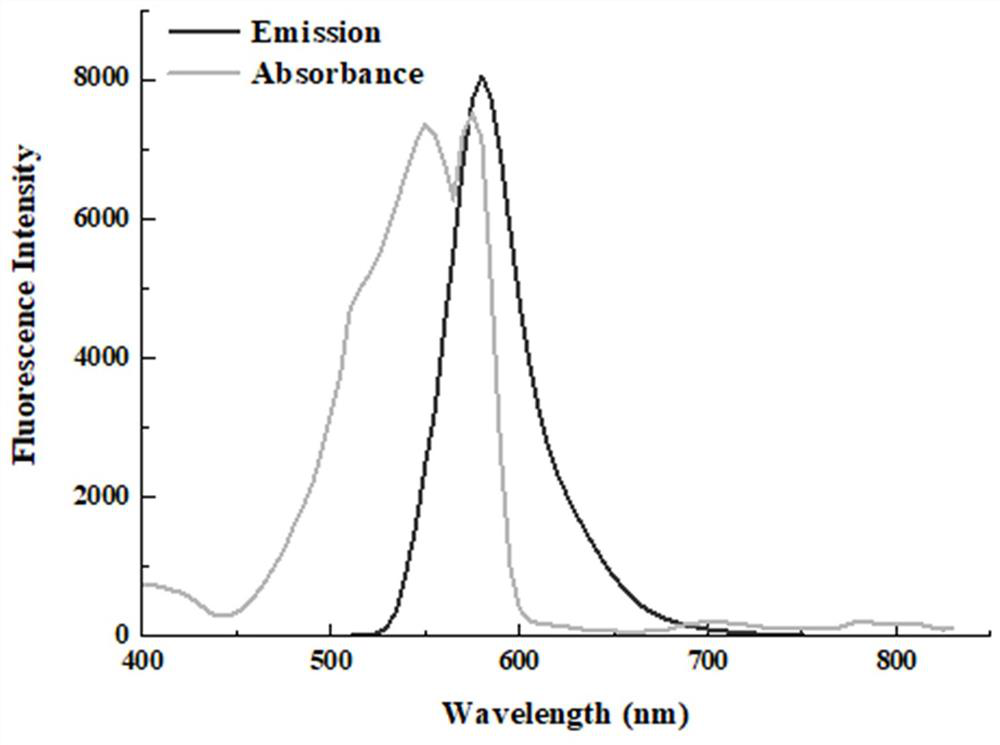
[0042] 2) Take 200 μL of 0.1 mM fluorescent probe Rho-IDA-CoII aqueous solution into a micro-quartz cuvette, and use a UV-visible light spectrophotometer to scan the Rho-IDA-CoII sample at a full wavelength, with a wavelength range of 200 nm~ 850nm, with ultrapure water as a blank control. The results of full-wavelength scanning showed that the fluorescent probe Rho-IDA-CoII had maximum absorption peaks at two wavelengths of 550 nm and 575 nm.
[0043] 3) Transfer the fluorescent probe Rho-IDA-CoII solution sample in step 2 to a micro-fluorescent cuvette, set the excitation wavelength to 550 nm, and scan the fluorescent probe Rho-IDA-CoII in the wavelength range of 400-750 nm Fluorescence intensity within;
[0044] 4) The test results show that ( i...
Embodiment 3
[0045] Example 3: In vitro staining of histidine-tagged proteins based on fluorescent probe Rho-IDA-CoII
[0046] 1) Mix the histidine-tagged fusion protein to be detected (SEQ ID NO. 1) with electrophoresis loading buffer at a ratio of 3:1, and bathe in boiling water for 5 min;
[0047] 2) Use 4-20% gradient gel (GenScript, China) to separate the protein samples by SDS-PAGE, the electrophoresis condition is 200 V constant voltage, 40 min;
[0048] 3) After electrophoresis, carefully soak the gel in fixative solution for 1 h;
[0049]4) Rinse the gel twice with deionized water, 10 min each time, to fully remove the fixative;
[0050] 5) Transfer the gel to fluorescent staining solution and stain in the dark for 1 h;
[0051] 6) Rinse the gel twice with washing solution, 10 min each time;
[0052] 7) Use Bio-Rad CCD camera to capture fluorescent gel images;
[0053] 8) Stain the gel that has recorded the fluorescent staining results in step 7 with Coomassie Brilliant Blue, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com