Molybdenum-based electrocatalyst, preparation method of molybdenum-based electrocatalyst, difunctional electrolytic tank and application of difunctional electrolytic tank
An electrocatalyst and electrolytic cell technology, which is applied in the field of hydrogen production, can solve the problems of unstudied electrolytic cell voltage, complex process and device, and high cost, and achieve the effects of improving hydrogen production efficiency, low cost, and high electrolysis efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] At room temperature, add 1.23g of ammonium molybdate and 0.4g of polyethylene glycol into a beaker filled with 30mL of water, and stir for 30min; then add 5ml of nitric acid with a concentration of 65%-68%, stir for 30min, and transfer to In a polytetrafluoroethylene lining, hydrothermal reaction at a constant temperature of 160°C for 24 hours; after the reaction is complete, naturally cool to room temperature, wash with ethanol and water, centrifuge, and dry, and the product is a molybdenum-based electrocatalyst α -MoO 3 .
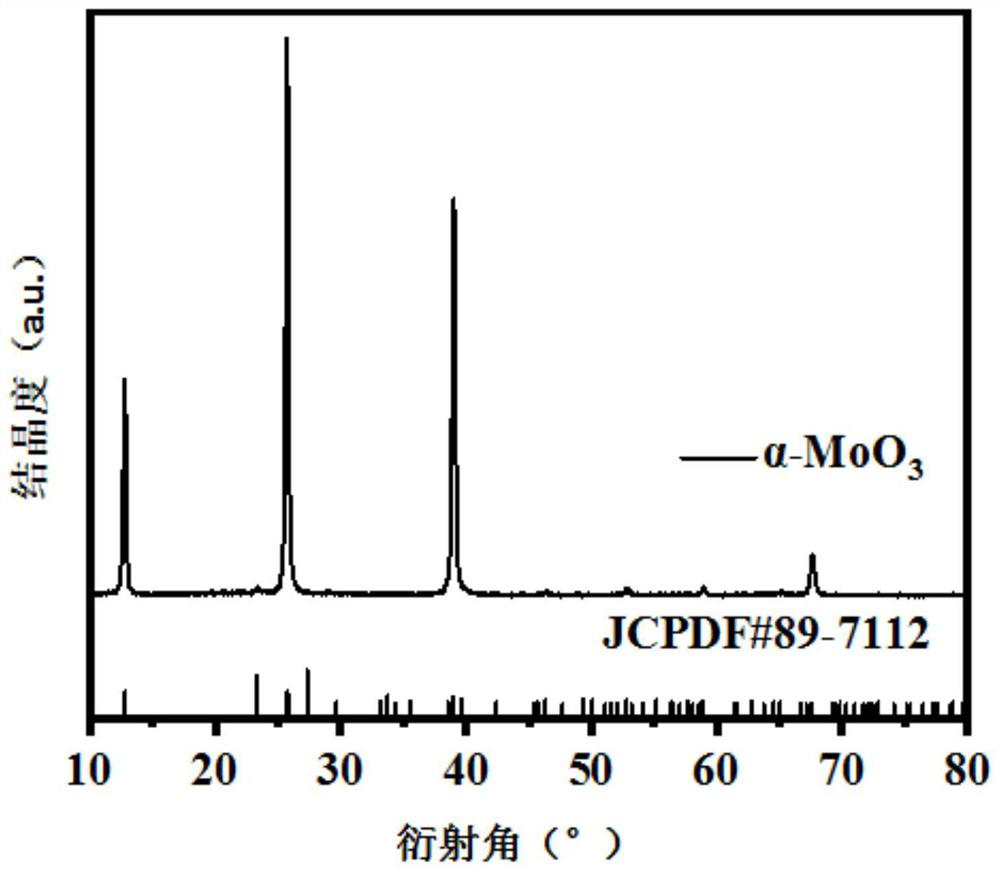
[0028] figure 1 Prepared for this example α -MoO 3 The X-ray diffraction (XRD) pattern, as can be seen from the figure, the product prepared in this embodiment is the same as α -MoO 3 The standard card (JCPDS: 89-7112) is consistent, indicating that the prepared product is phase-pure α -MoO 3 electrocatalyst.
[0029] figure 2 Prepared for this example α -MoO 3 Scanning electron microscope (SEM) figure, it can be seen from the figure th...
Embodiment 2
[0031] At room temperature, add 1.2g of ammonium molybdate and 0.4g of polyethylene glycol into a beaker filled with 30mL of water, and stir for 30min; then add 5ml of nitric acid with a concentration of 65%-68%, stir for 30min, and transfer to In a polytetrafluoroethylene lining, hydrothermal reaction at a constant temperature of 160°C for 24 hours; after the reaction is complete, naturally cool to room temperature, wash with ethanol and water, centrifuge, and dry, and the product is a molybdenum-based electrocatalyst α -MoO 3 , its XRD figure and SEM figure are identical with embodiment 1.
Embodiment 3
[0033] At room temperature, add 1.20g of ammonium molybdate and 0.387g of polyethylene glycol into a beaker containing 28mL of water, and stir for 20min; then add 4.9ml of nitric acid with a concentration of 65%-68%, stir for 20min, and transfer Into the polytetrafluoroethylene lining, hydrothermal reaction at a constant temperature of 170 ° C for 22 hours; after the reaction is complete, naturally cool to room temperature, wash with ethanol and water, centrifuge, and dry, the product is molybdenum-based electrocatalyst α -MoO 3 , its XRD figure and SEM figure are identical with embodiment 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com