Medicine for treating myocardial ischemia and preparation method thereof
A pharmacy and prodrug technology, applied in the field of medicine, can solve the problems of adverse reactions, uncertain curative effect, and high dependence on individual physique of patients, and achieve the effects of outstanding drug efficacy, reduction of myocardial infarction area, and high product yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
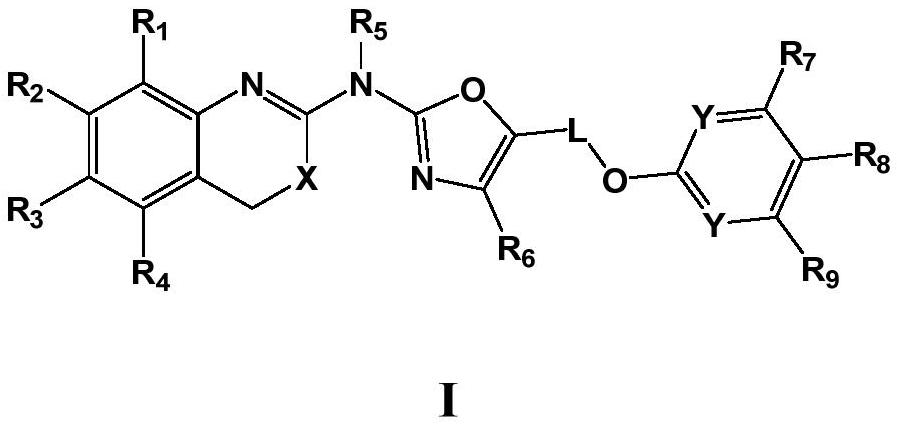
[0081] In the fifth aspect of the present invention, the present invention also provides the preparation method of the compound of formula I of the present invention, which comprises the following steps:
[0082]
[0083] Step 1: the compound of formula II reacts with the compound of formula III to generate the intermediate compound of formula IV;
[0084] Step 2: the intermediate compound of formula IV reacts with the compound of formula V to generate the compound of formula I;
[0085] Among them, X a 、X b Each independently represents halogen, preferably chlorine or bromine;
[0086] X, R 1 -R 9 , L, Y are as defined herein.
[0087] Preferably, step 1 is carried out under the action of a base; preferably, the base is selected from organic bases, especially triethylamine and pyridine.
[0088] Preferably, step 2 is carried out in the presence of cuprous salts, ligands, and alkalis; preferably, the cuprous salts are selected from cuprous bromide and cuprous iodide; ...
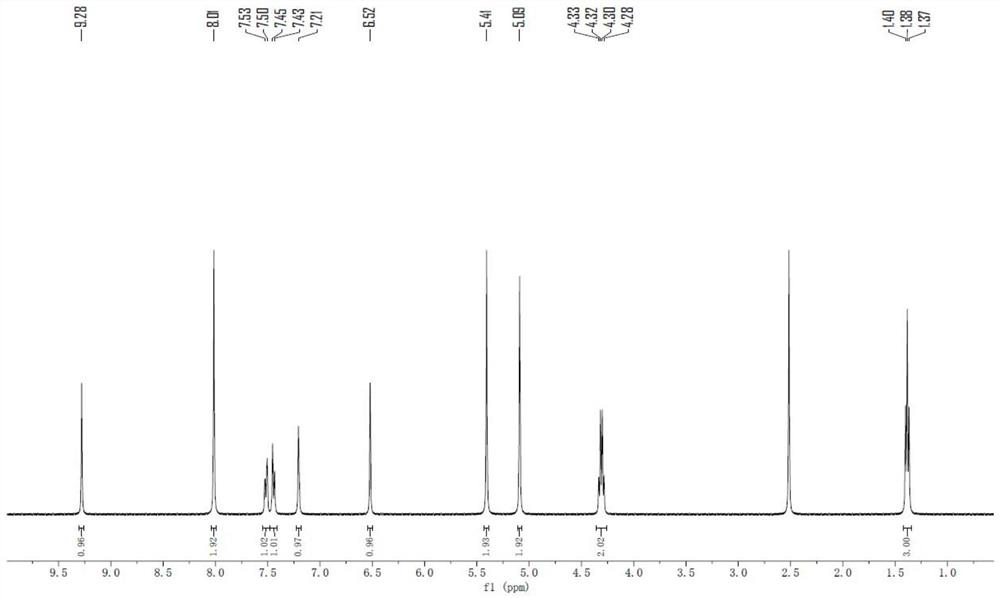
Embodiment 1
[0091] Embodiment 1: the preparation of compound 1
[0092]
[0093] Preparation of intermediate IV-1: under nitrogen protection, compound II-1 (7.11g, 30mmol) was dissolved in toluene (120ml), triethylamine (21ml) was added, compound III-1 (3.42g, 30mmol ) dissolved in toluene (50ml); then heated to 95 ° C for 2 hours. After cooling down to normal temperature, wash the reaction solution with water for several times, evaporate the solvent under reduced pressure, add chloroform to dissolve, filter and wash with saturated saline and water for several times, dry the organic phase with anhydrous sodium sulfate, and evaporate under reduced pressure The solvent was recrystallized with ethanol / diethyl ether (volume ratio 4:1), and dried to obtain 6.12 g of intermediate IV-1 with a yield of 75.6%. ESI-MS: 271.2[M+H] + .
[0094] Preparation of compound 1: under nitrogen protection, intermediate IV-1 (4.05g, 15mmol), compound V-1 (2.38g, 15mmol), cuprous iodide (0.95g, 5mmol), ce...
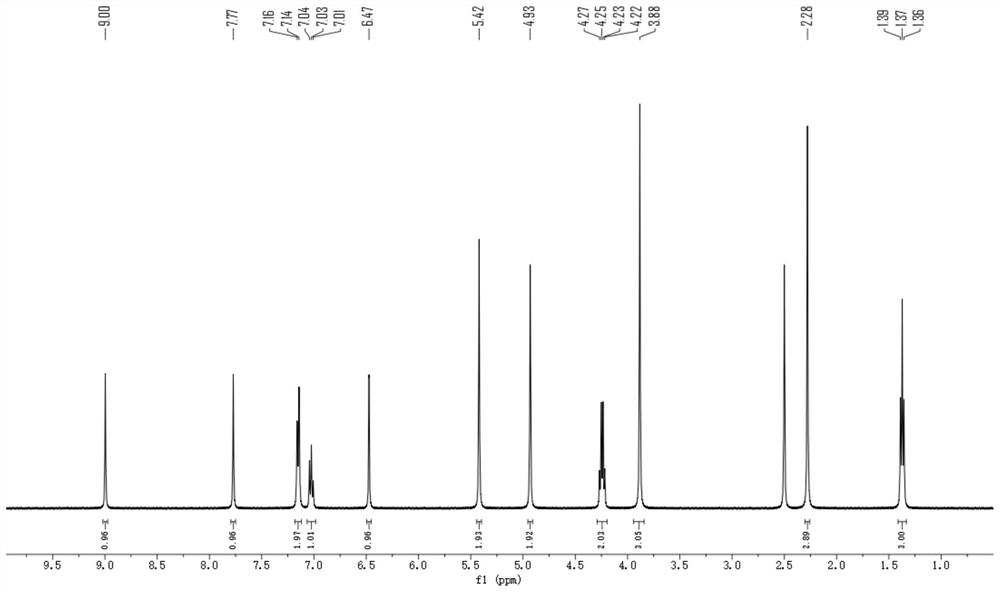
Embodiment 2
[0098] Embodiment 2: the preparation of compound 2
[0099]
[0100] According to the same preparation method as in Example 1, compound II-1 was replaced by equimolar II-2, and intermediate IV-1 was replaced by equimolar IV-2 to obtain compound 2 (4.30g), HPLC purity≧98.9 %.
[0101] ESI-MS: 386.2[M+H] + .
[0102] Elemental analysis: theoretical element content (%) C 18 h 16 FN 5 o 4 : C, 56.10; H, 4.19; N, 18.17; Measured element content (%): C, 56.13; H, 4.22; N, 18.15.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com