Preparation method of phosphine oxide type TADF organic photoelectric material
An organic optoelectronic material, phosphine oxide technology, applied in the direction of luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve the problems of difficult processing, difficult manufacturing process, low efficiency, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
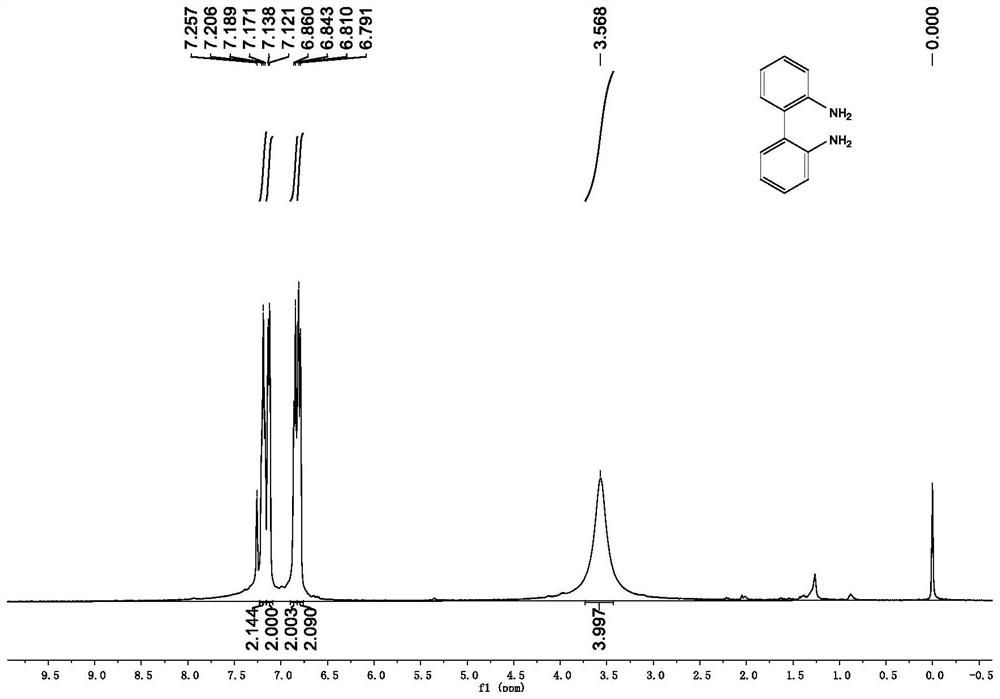
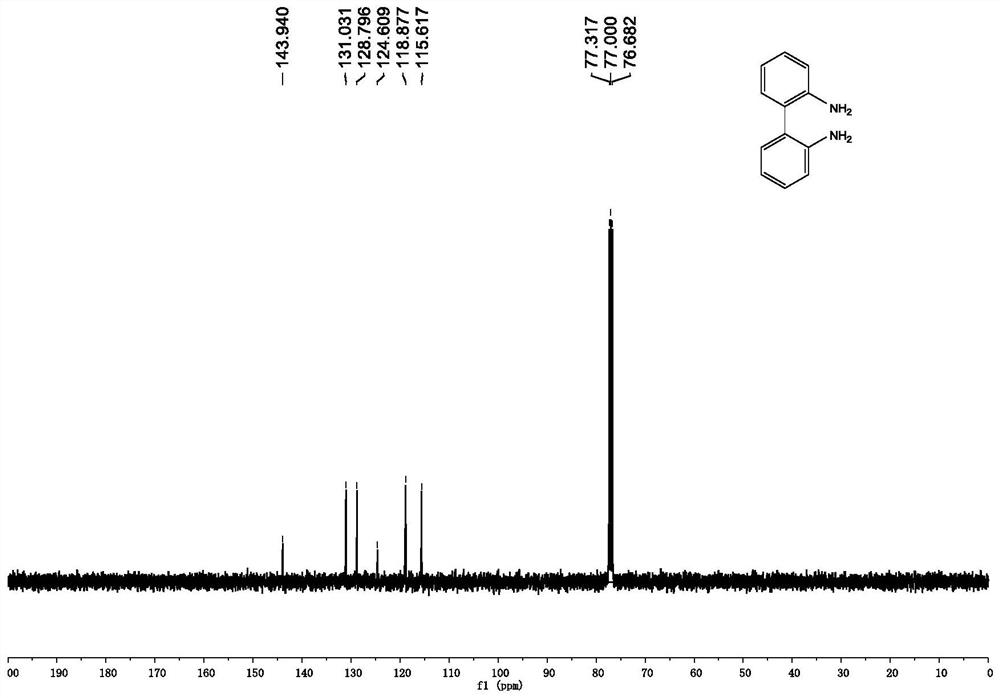
[0046] Synthesis of 2,2'-diamino-1,1'-biphenyl:
[0047] Under nitrogen protection, add 40mmol (1.0eq) 2,2'-dinitro-1,1'-biphenyl, 50mL ethanol and 50mL 8M hydrochloric acid mixed solution into a 250mL single-necked flask equipped with a magnet, and wait until the mixture is fully dissolved , add 240mmol tin dichloride (6.0eq) to the reaction system in batches, keep the temperature of the mixture at 100° C. and continue stirring for 6 hours after the addition is complete. After the reaction is complete, the mixed solution is cooled to room temperature, ethanol is distilled off under reduced pressure, NaOH solution is added to the residual solution, and the pH of the solution is adjusted to 8-10, then the mixture is extracted three times with ethyl acetate, the organic phases are combined and sequentially washed with saturated Wash with sodium bicarbonate and saturated brine, dry the organic phase with anhydrous sodium sulfate, filter, and remove the solvent under reduced press...
Embodiment 2
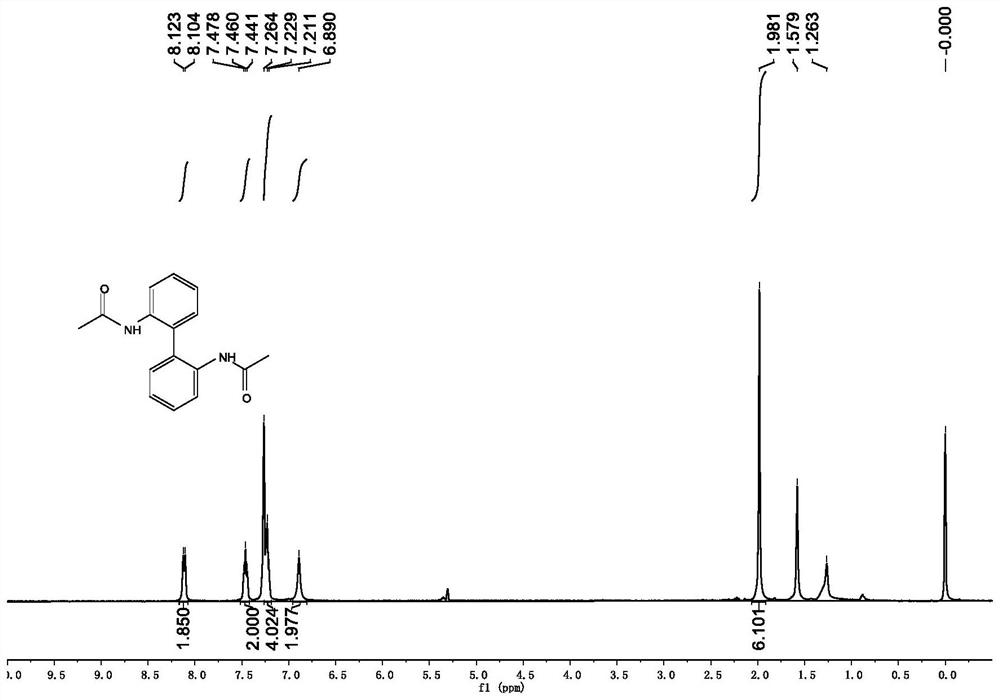
[0049] Synthesis of 2,2'-diacetamido-1,1'-biphenyl:
[0050] Under the protection of nitrogen, put 34.5mmol (1.0eq) 2,2'-diamino-1,1'-biphenyl, 100mL dichloromethane and 90mmol triethylamine into a 250mL flask equipped with a magnet, and after the addition The mixture was cooled in an ice-water bath environment, and then 78 mmol of acetyl chloride was slowly added dropwise to the reaction system with a syringe. After the dropwise addition, the reaction temperature was slowly raised to room temperature and the reaction was stirred overnight. After the reaction was complete, the reaction was quenched with saturated ammonium chloride, the mixture was extracted three times with dichloromethane, the organic phases were combined and washed with saturated sodium chloride, dried over anhydrous sodium sulfate, filtered, and the solvent was removed under reduced pressure to obtain 2,2'- Diacetamido-1,1'-biphenyl 4, yield 93%.
Embodiment 3
[0052] Synthesis of 5,5'-dibromo-2,2'-diacetamido-1,1'-biphenyl:
[0053] Under nitrogen protection, 8.48mmol (1.0eq) of 2,2'-diacetamido-1,1'-biphenyl and 24mL of DMF were added to a 100mL flask equipped with a magnet, and then 18.66 mmol NBS, after the addition was complete, the reaction was stirred overnight at room temperature. After the raw materials are completely reacted, add an appropriate amount of water to the reaction system and a large amount of white solid precipitates out, filter, and wash the filter cake with water and petroleum ether in turn, and the obtained dry filter cake is the target product 5,5'-dibromo-2 ,2'-Diacetamido-1,1'-biphenyl, the yield is 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com