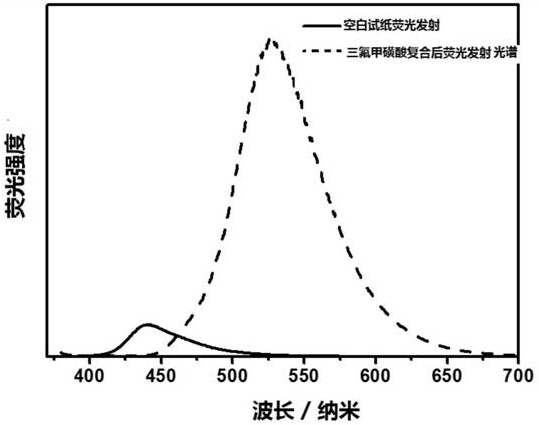
Organic acid property detection agent and method, test paper, preparation method and application
A technology for detecting test strips and detection agents, which is applied in organic chemistry, analysis by chemical reaction of materials, and material analysis by observing the influence of chemical indicators. It can solve problems such as lack of strong acid gas detection, and achieve easy Observation, synthesis method is simple, the effect of early warning leakage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
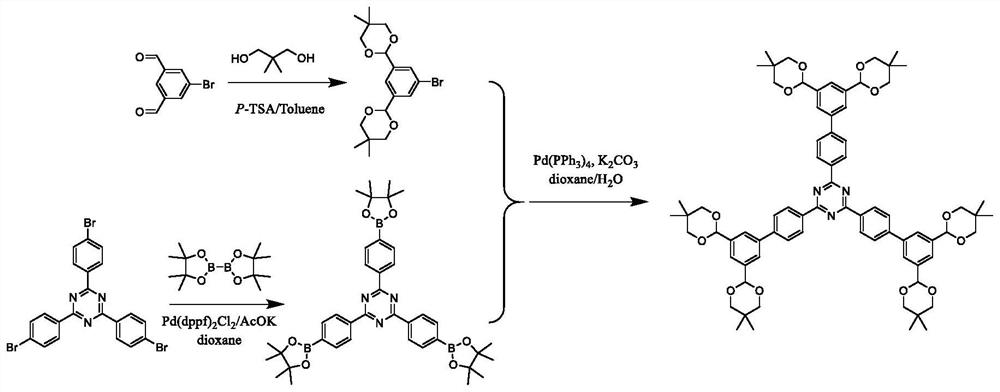
[0035] A method for preparing an organic acid detection agent, said method comprising the steps of:
[0036] Under the action of p-toluenesulfonic acid, 5-bromo-isophthalaldehyde is condensed with neopentyl glycol, and the corresponding 5-bromo-isophthalaldehyde is obtained through a chromatographic column ,3-dioxane compound;
[0037] Wherein, the feeding ratio of 5-bromo-isophthalaldehyde and neopentyl glycol is 1:1~5;
[0038] Synthesize 1,3,5-tris(bromophenyl)-pyrazine and bipinacol borate under the action of an iron catalyst to synthesize the corresponding 1,3,5-tris(pinacol borate)- pyrazine;
[0039] Wherein, the iron catalyst includes 1,1'-bisdiphenylphosphinoferrocene.
[0040] The obtained 1,3,5-tris(pinacol borate)-pyrazine and 5-bromo-isophthal-2-5,5-dimethyl-1,3-dioxane compound in palladium catalyst The corresponding 1,3,5-tris(5,5-dimethyl-1,3-dioxane biphenyl)-pyrazine can be obtained by carbon-carbon coupling under the action.
[0041] Wherein, the pallad...
Embodiment 1
[0053] (1) Preparation of intermediate 5-bromo-resorcinol-5,5-dimethyl-1,3-dioxane ( figure 1 )
[0054] Weigh 5-bromo-isophthalaldehyde (0.1mmol) and neopentyl glycol (injection amounts are 0.1, 0.2, 0.3, 0.4, 0.5mmol respectively) and put it into a single-necked bottle with 50mL of toluene solvent, and quickly add Catalytic amount (0.005mmol) of p-toluenesulfonic acid was heated to 120 degrees Celsius and continued to reflux for 12 hours. After the reaction, the reaction solution was cooled. The toluene in the reaction bottle was removed by rotary evaporation, dissolved in dichloromethane, mixed with silica gel, dry-loaded, and purified by column using petroleum ether as a developer to obtain the intermediate intermediate 5-bromo-resorcin-5, For 5-dimethyl-1,3-dioxane, the yields obtained by the corresponding dosages were 30%, 50%, 82%, 85% and 91%.
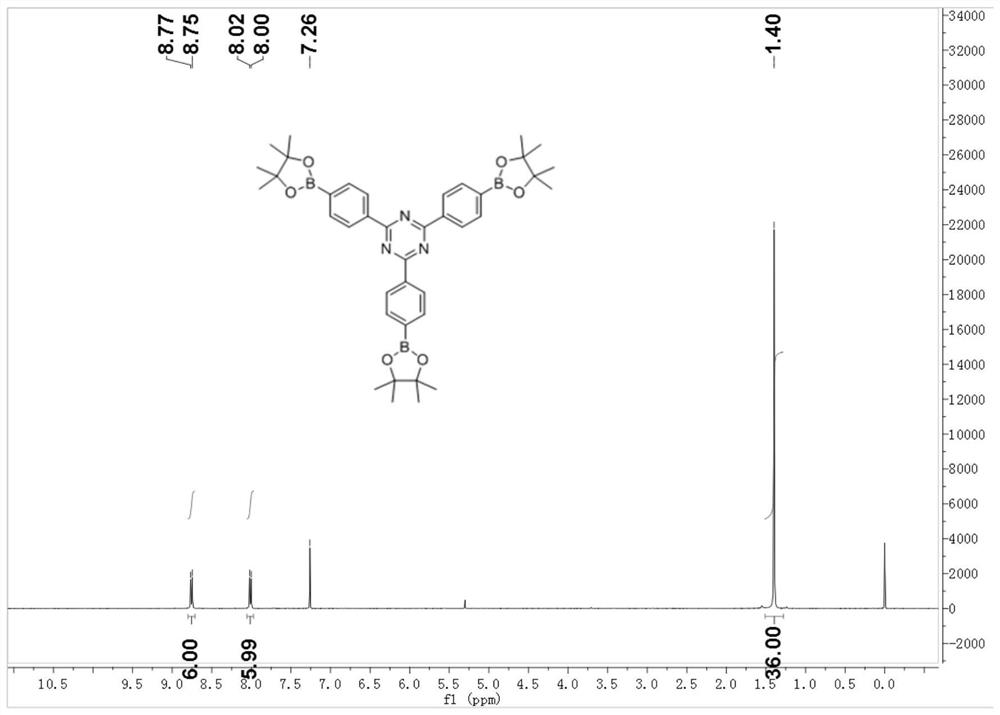
[0055] (2) Preparation of 1,3,5-three (pinacol borate)-pyrazine ( figure 1 )
[0056]Put 1,3,5-tris(bromophenyl)-pyrazin...
Embodiment 2
[0060] Preparation method of organic acidic solvent test paper with 1,3,5-tris(5,5-dimethyl-1,3-dioxanebiphenyl)-pyrazine as active ingredient (including models I, II and III )
[0061] 1,3,5-tris(5,5-dimethyl-1,3-dioxane biphenyl)-pyrazine (0.1mmol) and 1,4-butanediol (injecting amounts of 0.1, 0.2,0.3mmol) into the single-necked bottle that the mixture of polytetrahydrofuran diol and polyethylene oxide diol is housed, heated to 70 degrees Celsius, reacted for 2 hours. Put 3,3'-dichloro-4,4'-diphenylmethanediamine (0.1 / 0.2 / 0.3mmol) into the above one-necked bottle, and continue to react for 8 minutes. After the reaction is completed, spread the reaction solution evenly on the On the original pulp paper, dry for 24 hours to obtain three models ( I, II and III) organic acid solvent detection test paper.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com