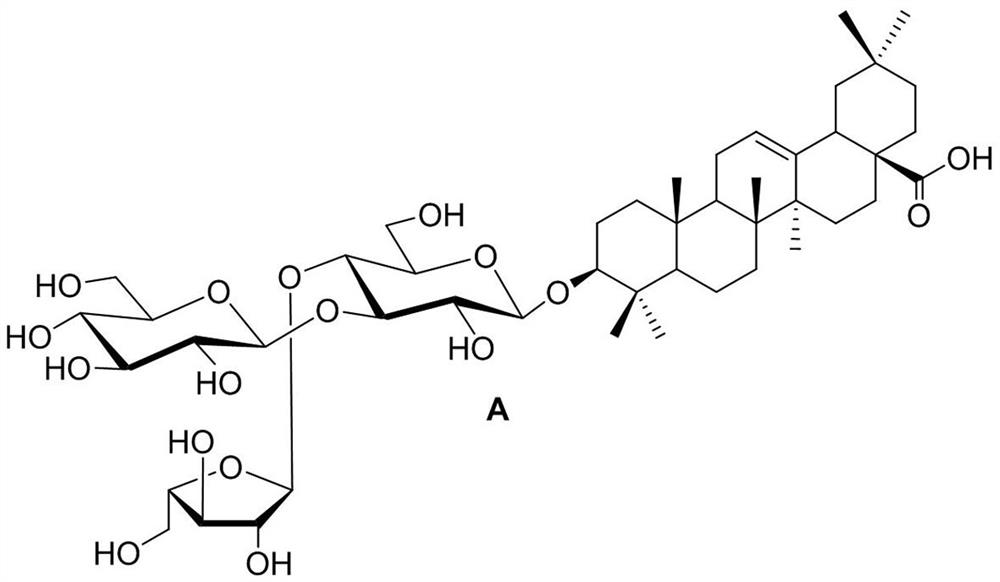
Novel panax stipuleanatus saponin analogue as well as synthesis method and application thereof
A technology of notoginseng saponins and analogues, applied in the field of synthesis of novel notoginseng saponins analogues, can solve problems such as resource difficulties, achieve high yields, solve drug resistance problems, and achieve short routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
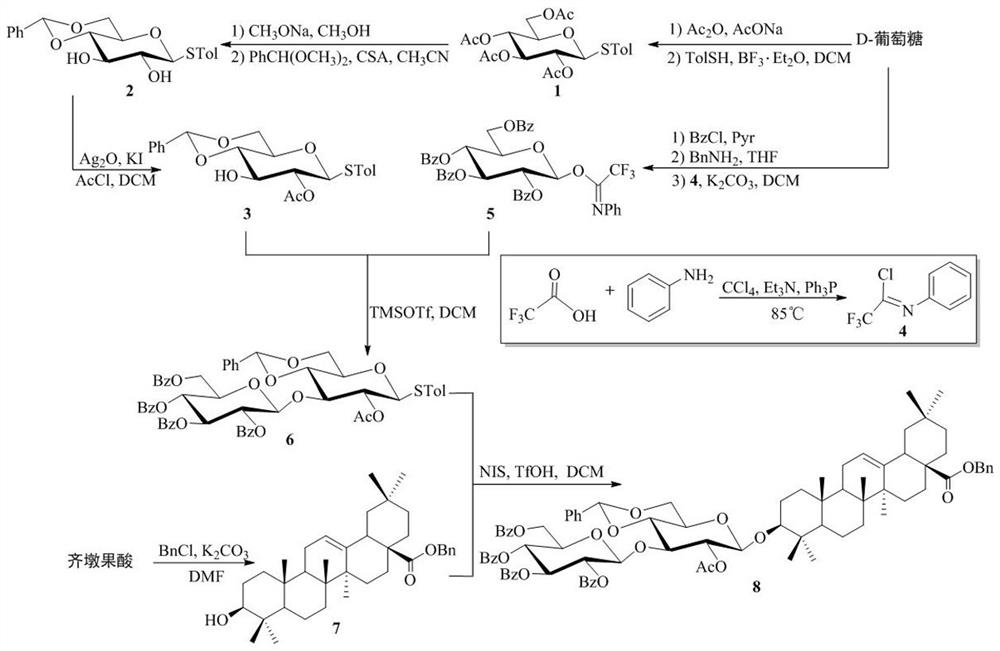
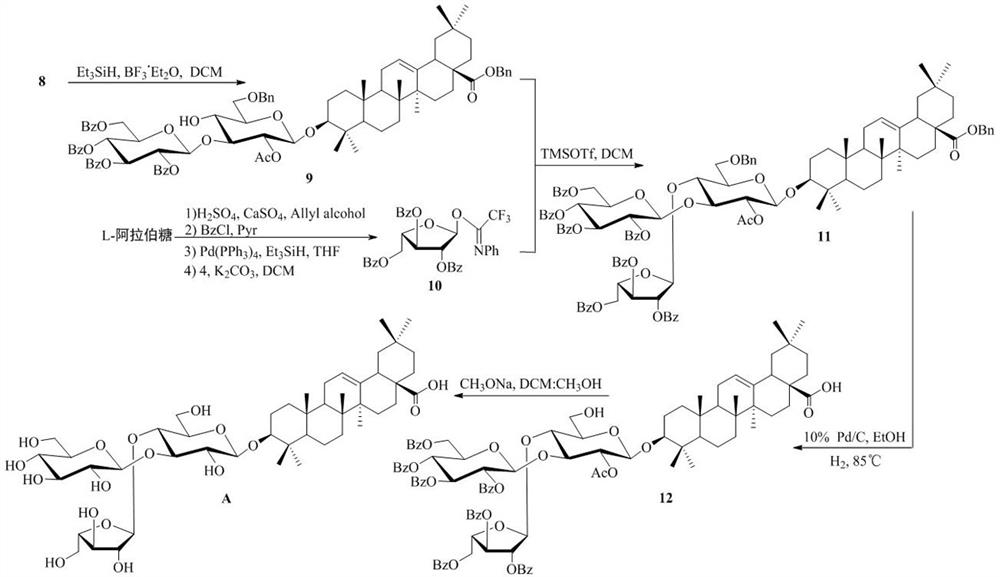
[0030] Synthesis method of novel screenside pentaseng saponin analogues, including (1) synthesis of disaccharide saponin 8 and (2) synthesis of new screenside pentaseng saponin analogue A, the specific steps are:
[0031] (1) Synthesis of disaccharide saponin 8: taking D- glucose as the starting material, after full acetylation, synthesizing thioside 1 with p-toluenethiophen under the action of boron trifluoride diethyl ether of Lewis acid; thioside 1 is hydrolyzed under alkaline conditions of sodium methanol, and the synthetic compound 2 is protected by benzyl fork, and the 2-hydroxy of acetyl selective protective compound 2 is obtained to the glycosylthioside receptor 3; after D-glucose perbenzoylation, the glucose trifluoroacetimide ester donor is synthesized with N-phenyl trifluoroacetimide chloride 4 Glycosacylate receptor 3 and glucose trifluoroacetimide donor 5 were given disaccharide 6 under the action of trimethylsilyl trifluoromethanesulfonate; oleanolic acid reacted wit...
Embodiment 1
[0037] Synthesis method of novel screenside pentaseng saponin analogue A
[0038] Synthesis of thioside (1).
[0039]
[0040] Add NaOAc (12.2 mmol, 1.0 g) with Ac in a dry round-bottom bottle 2 O(99.4 mmol, 9.4 mL), heated to reflux, D-glucose (10 mmol, 1.8 g) was added in 3 times within 15 min, reflux stirring reaction for 4 h, thin layer chromatography detection reaction was complete. The reaction liquid was cooled to room temperature, crushed ice was added, and the white solid was quickly precipitated by sonication. Filtered, washed the filter cake with water until no acidity, dissolved in hot ethanol (5 mL), added petroleum ether (100 mL), cooled at low temperature overnight to precipitate the white solid, filtered, petroleum ether washed filter cake, to give a white solid 1,2,3,4,6-five- O -Acetyl-β-D-glucose. Weigh 1, 2, 3, 4, 6-Five- O -Acetyl-β-D-glucose (2.6 mmol, 1.0 g), 4Å molecular sieve, TolSH (5.2 mmol, 0.6 g), soluble in dried DCM (10 mL). Cool to 0 °C, N 2 Slowl...
Embodiment 2
[0089] Take a little new screen edge panax notoginseng saponin analogue A, screen edge panax notoginseng total saponin and mixture of the two, add methanol to dissolve, respectively, on the same thin layer of chromatography plate, with dichloromethane - methanol - water as the unfolding agent to unfold, dry, spray with orcinol color developer, baked and dried on a 105 °C electric heater, inspected in daylight, thin layer chromatography as seen Figure 7 For the novel screen edge of the pentaseng saponin analogue A. Among them, 68 is a new type of screenside trisax pentaseng saponin analog, totally screenside triax notoginseng total saponin, mixed with a new type of screenside pentaseng saponin analogue A and screenside panax notoginseng total saponin mixture. As can be seen from the figure, the screen edge of the total saponin does not contain the new screen edge of the panax notoginseng saponin analogue A.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com