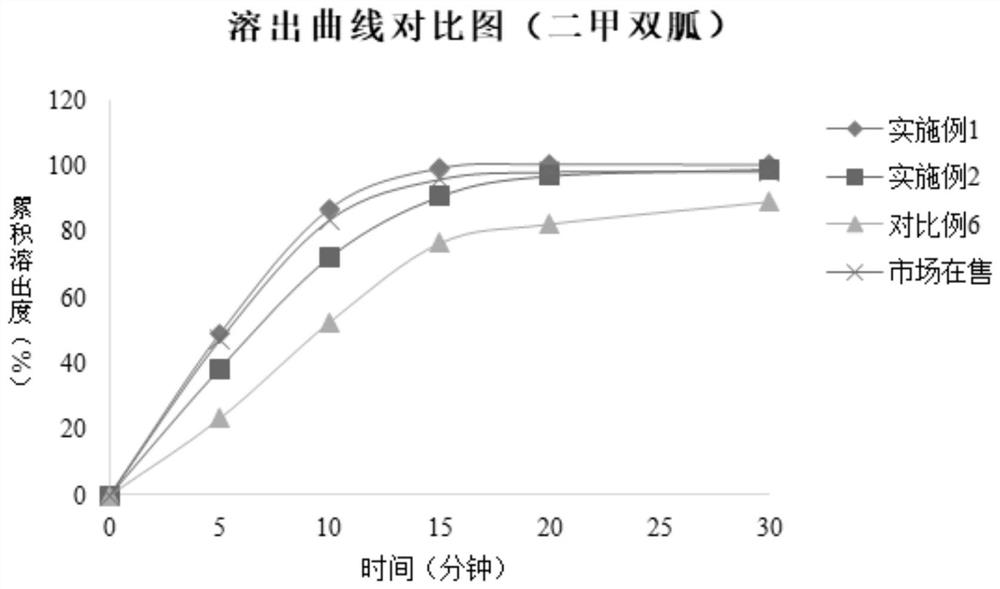
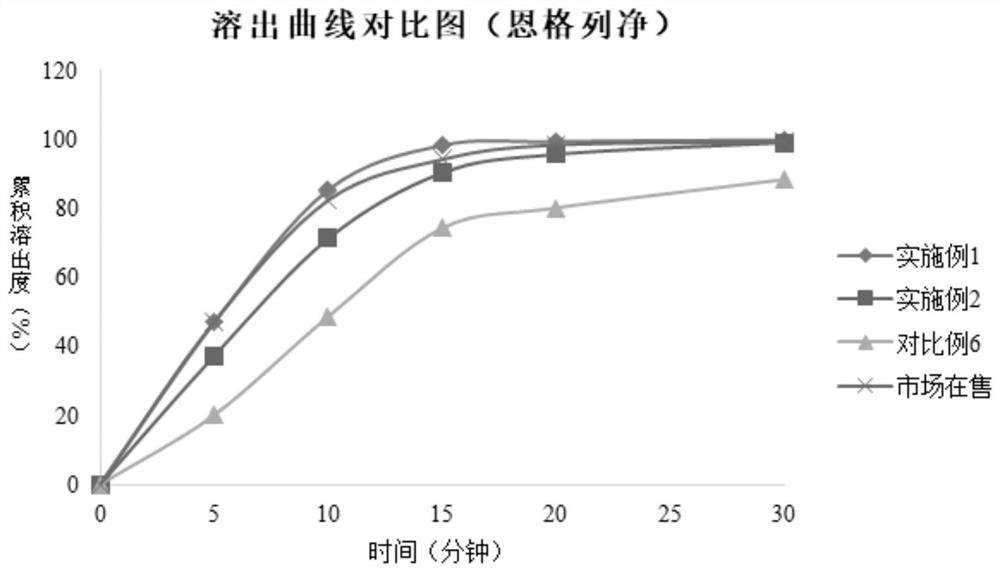
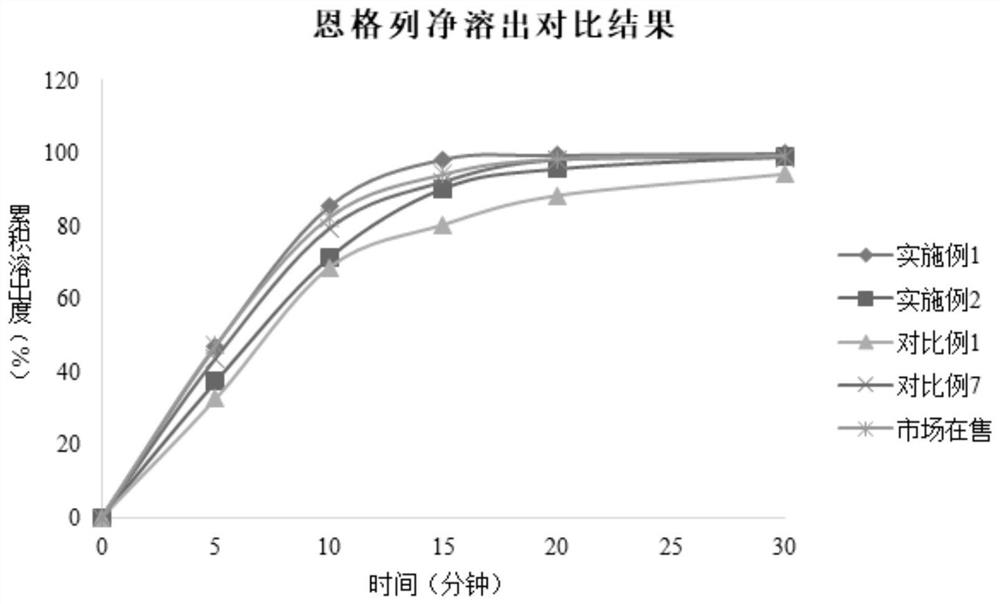
Metformin empagliflozin composition and preparation method thereof
A technology of empagliflozin and metformin, applied in the field of medicine, can solve the problems of poor content uniformity of empagliflozin tablets, unqualified friability, low tablet hardness, etc., and achieves qualified friability and fast dissolution rate , good compressibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1, Metformin Empagliflozin Tablets (500 / 5mg) preparation process:
[0036] Metformin Empagliflozin Tablets Prescription Composition
[0037]
[0038] Metformin Empagliflozin Tablets Preparation Method:
[0039] (1) Pretreatment of excipients: Use a universal pulverizer to crush and sieve the bulk drug of Empagliflozin to 80 meshes, sieve metformin hydrochloride and corn starch to 40 meshes, and sieve colloidal silicon dioxide to 80 meshes;
[0040] (2) Excipient premixing: put metformin hydrochloride and cornstarch in a column hopper mixer and mix them at a speed of 8r / min, and mix for 10min. Metformin hydrochloride;
[0041] (3) Fluidized bed granulation:
[0042] a. Preparation of granulation solution: dissolve copovidone in purified water to obtain a copovidone solution with a concentration of 21.2% (w / w), then slowly add empagliflozin raw material, stir and disperse evenly, and obtain drug-containing granules liquid, spare;
[0043] b. Granulation...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com