Vinyl bridged two-dimensional covalent organic framework material based on 2, 4, 6-trimethylpyridine and preparation method of vinyl bridged two-dimensional covalent organic framework material
A technology of covalent organic framework and collidine, which is applied in the preparation of organic compounds, organic chemistry, carboxylic acid ester preparation, etc., can solve the problems of complex monomer synthesis, tedious experimental steps, expensive reaction reagents, etc., and achieve high Chemical stability, avoidance of experimental steps, effects of high specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] In a glove box under an argon atmosphere, 121.18 mg of 2,4,6-collidine, 201.20 mg of terephthalaldehyde (or 393.40 mg of 1,3,5-tris(4'-formylphenyl)tri oxazine), 90.08 mg acetic acid and 153.13 mg acetic anhydride were added to a 5 mL ampoule. In the liquid nitrogen bath, the ampoule was sealed with a vacuum flame by means of cooling, evacuating and supplementing nitrogen, and transferred it to a muffle furnace, and heated to 180° C. for 72 hours to react. After the reaction, the reaction bottle was naturally cooled to room temperature, and the filter residue was collected by vacuum filtration, rinsed with acetone, methanol and methylene chloride, and extracted with tetrahydrofuran and methanol by Soxhlet, and the product was collected and vacuum-dried at 60°C for 12 hours , an orange solid was obtained.
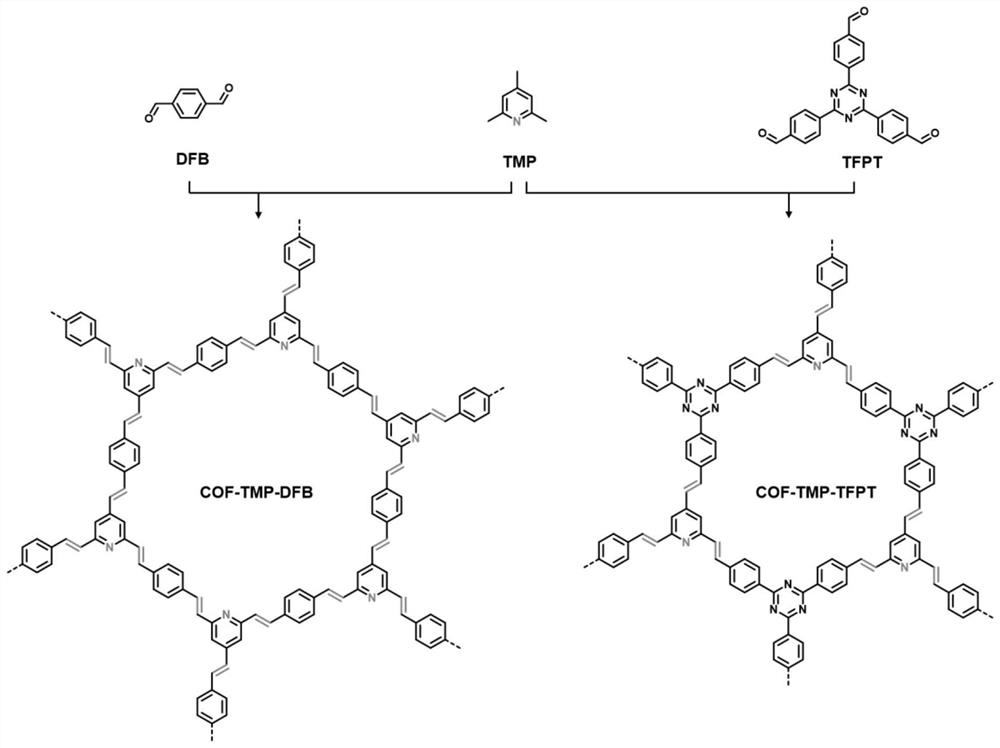
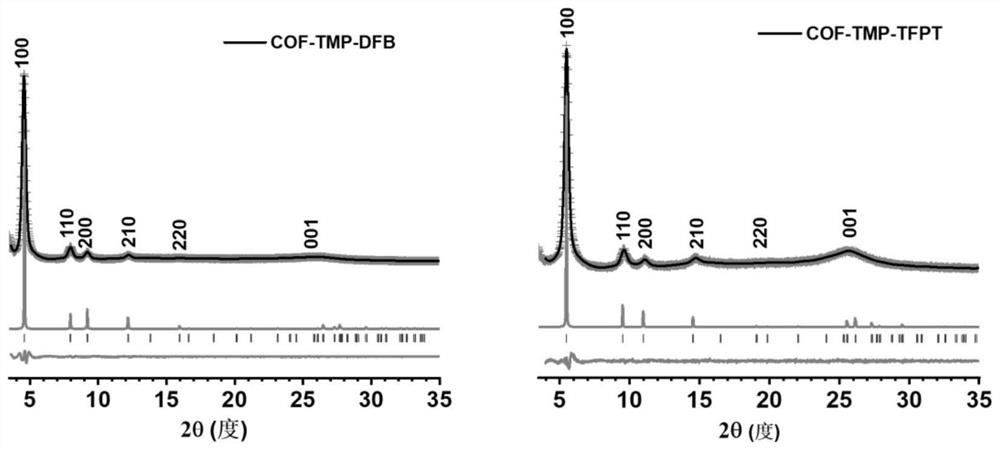
[0040] Schematic diagram of the synthesis and structure of two 2,4,6-collidine-based vinyl-bridged two-dimensional covalent organic frameworks figure 1 shown. The ...
Embodiment 2
[0047] In a glove box under an argon atmosphere, 121.18 mg of 2,4,6-collidine, 201.20 mg of terephthalaldehyde (or 393.40 mg of 1,3,5-tris(4'-formylphenyl)tri Zine), 210.85mg of benzoyl chloride and 216.16mg of sodium benzoate were added to a 5mL ampoule. In the liquid nitrogen bath, the ampoule was sealed with a vacuum flame by means of cooling, evacuating and supplementing nitrogen, and transferred it to a muffle furnace, and heated to 180° C. for 72 hours to react. After the reaction, the reaction bottle was naturally cooled to room temperature, and the filter residue was collected by vacuum filtration, rinsed with acetone, methanol and methylene chloride, and extracted with tetrahydrofuran and methanol by Soxhlet, and the product was collected and vacuum-dried at 60°C for 12 hours , an orange solid was obtained, and the products were named COF-TMP-DFB and COF-TMP-TFPT, respectively.
Embodiment 3
[0049] In a glove box under an argon atmosphere, 121.18 mg of 2,4,6-collidine, 201.20 mg of terephthalaldehyde, 60.05 mg of acetic acid, and 102.09 mg of acetic anhydride were added to a 5 mL ampoule. In the liquid nitrogen bath, the ampoule was sealed with a vacuum flame by means of cooling, evacuating and supplementing nitrogen, and transferred it to a muffle furnace, and heated to 180° C. for 72 hours to react. After the reaction, the reaction bottle was naturally cooled to room temperature, and the filter residue was collected by vacuum filtration, rinsed with acetone, methanol and methylene chloride, and extracted with tetrahydrofuran and methanol by Soxhlet, and the product was collected and vacuum-dried at 60°C for 12 hours , an orange solid was obtained with a yield of 79.3%. The product was named COF-TMP-DFB.
[0050] A magnetic stir bar was added to a 10mL thick-walled pressure-resistant tube, salicylic acid (1.0mmol, 138.12mg), acetic anhydride (2mL) and 30.0mg COF...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com