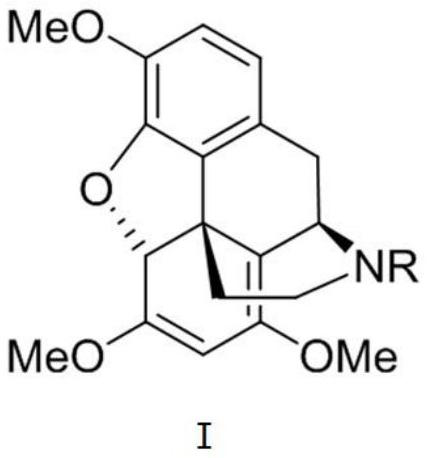
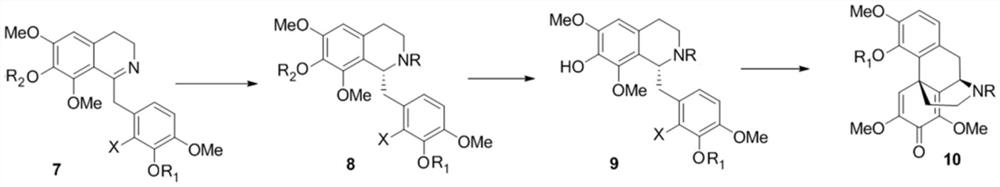
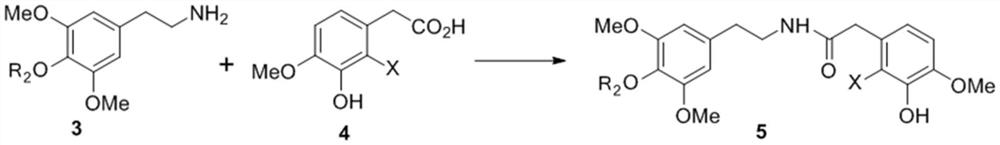
Intermediate as well as preparation method and application thereof
An intermediate and reaction technology, applied in the field of intermediates and their preparation, can solve the problems that the total synthesis method does not have practical significance and production value, the production process is complicated, the management and control process is long, etc., and the post-reaction treatment is simple and easy to operate and operate. Simple and effective in reducing the discharge of three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0246] The preparation method of embodiment 1 compound 3, synthetic route is:
[0247]
[0248] Include the following steps:
[0249] Compound 1 (71.3g, 0.317mol) was dissolved in 380mL of dichloromethane, imidazole (53.9g, 0.793mol) was added successively, DMAP (15.5g, 0.126mol) was stirred at room temperature, TBSCl (81.3g , 0.538mol), and stirred at room temperature for 1 h after addition. TLC detects that the reaction raw materials all disappear. use NH 4 The reaction was quenched with saturated aqueous Cl, and dichloromethane and water were added to the mixture until the solid precipitate was completely dissolved. The aqueous phase was extracted with ethyl acetate (200mL×5), the combined organic phases were washed with anhydrous Na 2 SO 4 Dry, filter and concentrate, add PE:DCM=10:1 (v / v) 200mL, stir evenly, filter, filter cake wash twice with PE:DCM=10:1(v / v), wash three times with PE and dry , to obtain bright yellow solid compound 2 (100 g, yield 93%).
[025...
Embodiment 2
[0253] The preparation of embodiment 2 compound 5, synthetic route is:
[0254]
[0255] Include the following steps:
[0256]Compound 4 (55.0g, 0.211mol), compound 3 (85.0g, 0.274mol) and TBTU (71.0g, 0.222mol) were placed in a 2L dry and clean round bottom flask, and after adding 750mL of dry dichloromethane to dissolve, the The reaction was placed in an ice bath with stirring and triethylamine (73 mL) was added. After the reaction was warmed to room temperature and stirred overnight, 1 L of saturated aqueous ammonium chloride solution was added to quench the reaction. The aqueous phase was extracted with dichloromethane (700 mL), and the combined organic phases were dried over anhydrous sodium sulfate, filtered, and concentrated. After the crude product was dissolved in 1L ethyl acetate, the organic phase was washed with 0.1M aqueous hydrochloric acid (350 mL×3). The organic phase was washed with saturated aqueous sodium bicarbonate (800 mL×2), dried over anhydrous so...
Embodiment 3
[0258] The preparation of embodiment 3 compound 6, synthetic route is:
[0259]
[0260] Include the following steps:
[0261] Compound 5 (60.6 g, 0.109 mol) was dissolved in 550 mL of acetonitrile, anhydrous potassium carbonate (45.2 g, 0.327 mol) and PMBCl (15.6 mL, 0.115 mol) were added in sequence, and the temperature was raised to 40° C. for 32 hours. TLC showed that the reaction of the raw materials was complete, and 21 mL of methylamine (2M / L methanol solution) was added to the reaction liquid, and stirred at room temperature for 2 hours. The mixture was filtered through celite, and the resulting filtrate was concentrated to obtain a crude product. The crude product was dissolved in 500 mL of ethyl acetate, the organic layer was washed with 0.1M hydrochloric acid aqueous solution (150 mL×3), saturated sodium bicarbonate aqueous solution (400 mL×2), dried over anhydrous sodium sulfate, filtered, and distilled under reduced pressure to obtain a light brown foam crude...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com