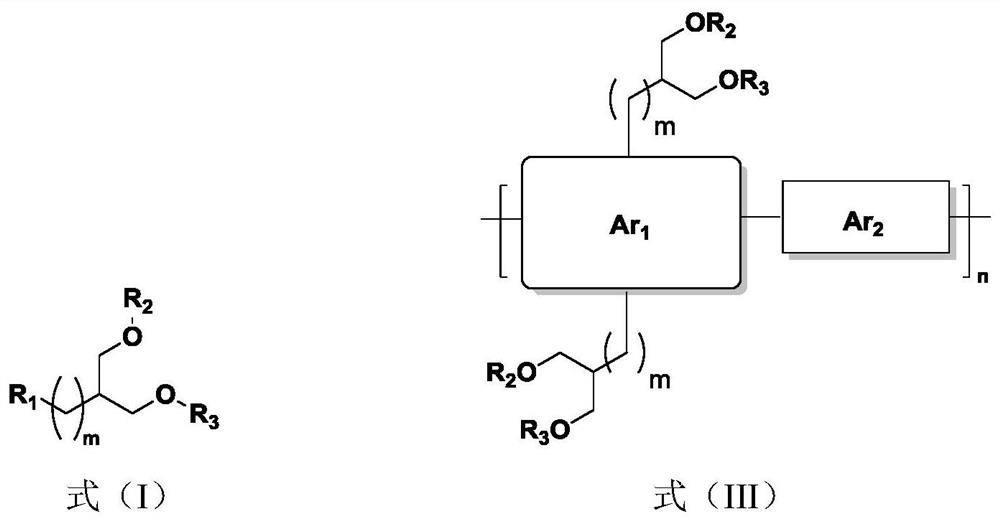
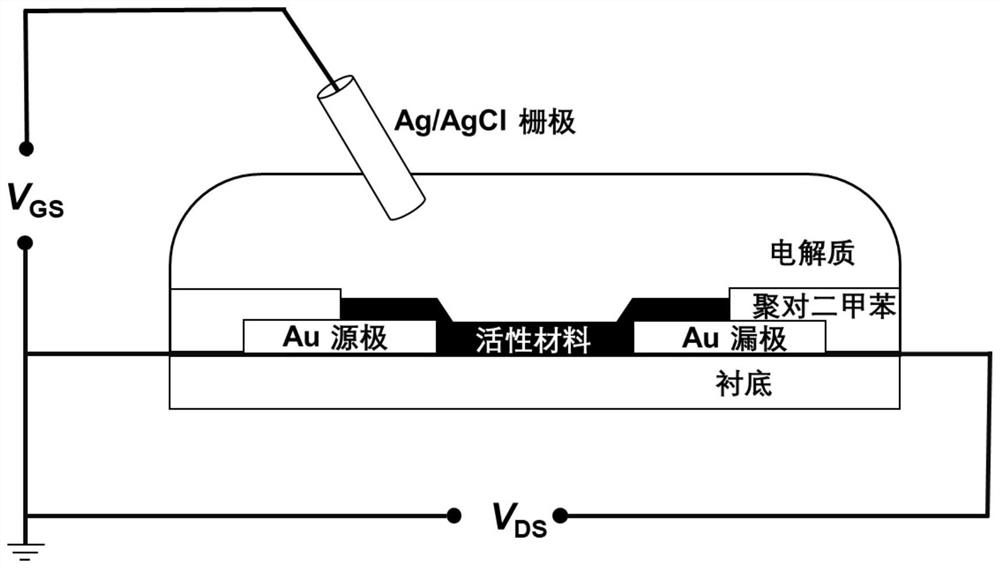
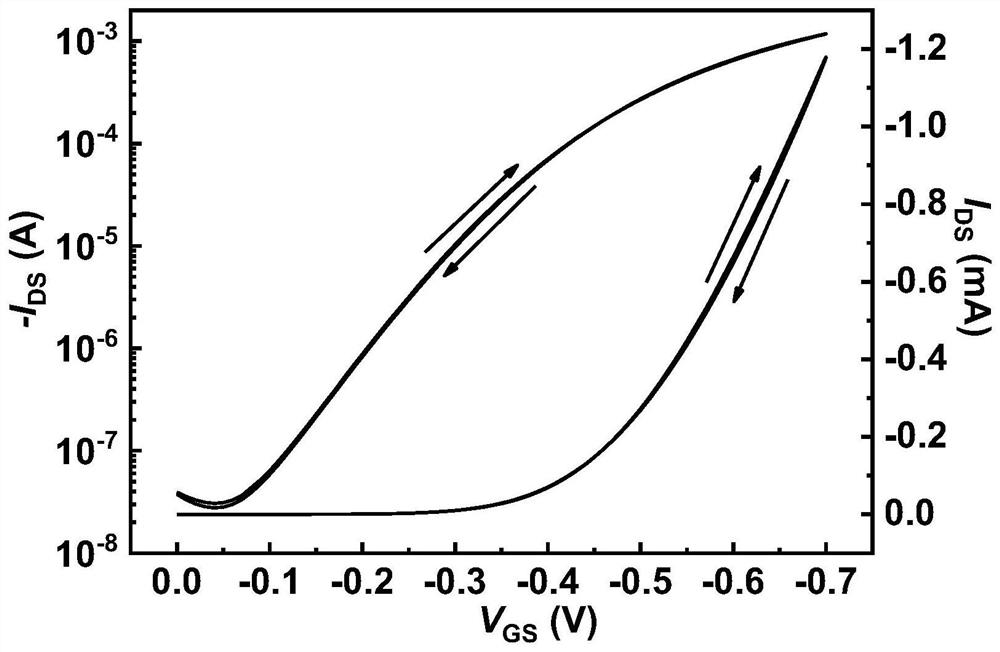
Organic conjugated molecule containing branched ether chain as well as preparation and application of organic conjugated molecule
A reaction and compound technology, applied in the field of organic polymer functional materials, can solve the problems of high toxicity of processing solvents and insufficient ion transport capacity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0118]
[0119] Synthesis process of compound 1: Add 5.24g of sodium hydride wrapped in 60% mineral oil to a 250mL dry double-necked bottle, replace the atmosphere of the system with nitrogen, add 120mL of dry tetrahydrofuran, and slowly dropwise add 27.4g of malonic acid Methyl ester, after the gas generation was completed, 25.0 g of benzyl bromopropyl ester was added dropwise, and the temperature was raised to 90 °C for reaction for 5 h. After the reaction, the heating was stopped, the system was cooled to room temperature, 50 mL of water was added to quench the reaction, the system was extracted with ether (50 mL×3), the organic phase was dried over anhydrous magnesium sulfate, filtered, and the solvent was removed by rotary evaporation. Distillation under reduced pressure gave 30.2 g of a colorless and transparent oily liquid with a yield of 99%. 1 H NMR (400MHz, Chloroform-d) δ7.27-7.37(m, 5H), 4.49(s, 2H), 3.73(s, 6H), 3.49(t, J=6.3Hz, 2H), 3.41(t, J=7.5Hz, 1H), 1.98...
Embodiment 2
[0121]
[0122] Synthesis route of compound 2: 0.84 g of lithium aluminum hydride was added to a 250 mL dry two-neck flask, and the system was replaced with nitrogen. Pour into dry 40 mL of tetrahydrofuran, slowly drop 2.56 g of compound 1 under an ice bath, and react at room temperature for 12 h. After the reaction was completed, 10 mL of saturated sodium sulfate solution was added dropwise in an ice bath, the system was passed through diatomaceous earth, the precipitate was washed with ethyl acetate, the mother liquor was collected, dried with anhydrous sodium sulfate, filtered, and the solvent was removed by rotary evaporation. Ester:dichloromethane=8:1 volume ratio of developing solvent column chromatography to obtain 1.69g of colorless and transparent oily product with a yield of 82%. 1 H NMR (400MHz, Chloroform-d) δ7.41-7.27(m, 5H), 4.49(s, 2H), 3.81-3.55(m, 4H), 3.47(t, J=6.4Hz, 2H), 2.80- 2.55(br,2H), 1.72(s,1H), 1.70–1.56(m,2H), 1.40–1.25(m,2H).
Embodiment 3
[0124]
[0125] Compound 3 synthesis route: add 0.45g sodium hydride wrapped in 60% mineral oil to a 250mL dry double-necked bottle, replace the system with a nitrogen atmosphere, add 30mL of tetrahydrofuran, and slowly dropwise add a tetrahydrofuran solution containing 1.06g of compound 2 , when the system no longer produces gas, drop 4.50g of MPEG3-OTs (triethylene glycol monomethyl ether p-toluenesulfonate), and react at 90°C for 12h. After the reaction was completed, heating was stopped, the system was cooled to room temperature, water was added to quench the reaction, the system was extracted with ether (50 mL×3), the organic phase was enriched, dried over anhydrous magnesium sulfate, filtered, and the solvent was removed by rotary evaporation. The residue was separated by column chromatography , the developing solvent is ethyl acetate:methanol=40:1 (volume ratio), and 0.80g of colorless and transparent oil was obtained by separation, and the yield was 33%. 1 H NMR (40...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com