Synthesis method of myosmine
A synthesis method and compound technology, applied in organic chemistry, bulk chemical production, etc., can solve the problems of high price of rhodium catalyst, no commercial production, lack of atom economy, etc., and achieve good application prospects and convenient product separation and purification. , the effect of good economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Embodiment 1, the preparation of mesmin
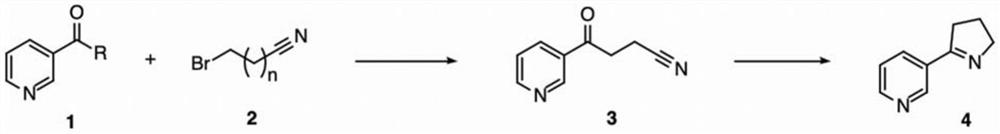
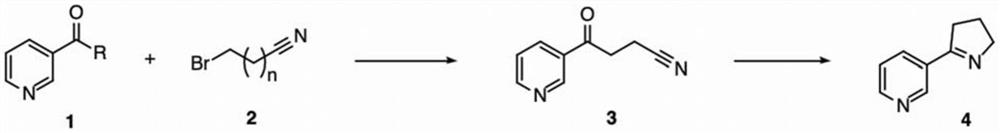
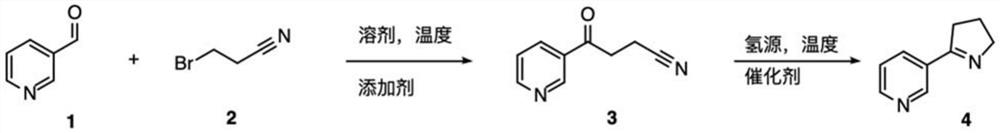
[0057] The reaction formula for preparing Mesmin by 3-pyridinecarboxaldehyde is as follows:
[0058]
[0059] Under nitrogen conditions, add iodine (0.01 mmol) and 3-bromopropionitrile (7.0 mmol) to the three-necked flask and dissolve in tetrahydrofuran (THF), place in an ice-water bath, add magnesium turnings (5.5 mmol) until the magnesium turnings dissolve, then , under the condition of ice-water bath, 3-pyridinecarboxaldehyde (5.0mmol) was slowly added dropwise to react for 2 hours, saturated ammonium chloride solution (20mL) was added to quench the reaction, ethyl acetate (20mL*3) was extracted, dried and concentrated to obtain the crude product , the crude product was dissolved in a single-necked flask with DCM, active manganese dioxide (60.0 mmol) was added and stirred at room temperature for 10 hours, filtered, and the filtrate was concentrated and spin-dried to obtain crude product 3, which was directly used in the ne...
Embodiment 2
[0061] Embodiment 2, the preparation of mesmin
[0062] Taking 3-pyridinecarboxaldehyde as raw material to prepare Maismin, the concrete method is as follows:
[0063] Under nitrogen conditions, iodine (0.01 mmol) and 3-bromopropionitrile (6.0 mmol) were added to the three-necked flask and dissolved in ether, placed in an ice-water bath, and magnesium turnings (6.0 mmol) were added until the magnesium turnings were dissolved. Under water bath conditions, 3-pyridinecarbaldehyde (5.0 mmol) was slowly added dropwise to react for 2 hours, saturated ammonium chloride solution (20 mL) was added to quench the reaction, extracted with ethyl acetate (20 mL*3), dried, and concentrated to obtain the crude product. Dissolve in a single-necked flask with DCM, add active manganese dioxide (50.0 mmol) and stir at room temperature for 10 hours, filter, concentrate the filtrate and spin dry to obtain crude product 3, which is directly used in the next reaction.
[0064] The crude product 3 wa...
Embodiment 3
[0065] Embodiment 3, the preparation of mesmin
[0066] Taking 3-pyridinecarboxaldehyde as raw material to prepare Maismin, the concrete method is as follows:
[0067] Under nitrogen conditions, add iodine (0.01 mmol) and 3-bromopropionitrile (6.0 mmol) to the three-necked flask and dissolve in THF, place in an ice-water bath, add magnesium turnings (6.5 mmol) until the magnesium turnings are dissolved, then, in ice Under water bath conditions, 3-pyridinecarbaldehyde (5.0 mmol) was slowly added dropwise to react for 3 hours, saturated ammonium chloride solution (20 mL) was added to quench the reaction, extracted with ethyl acetate (20 mL*3), dried, and concentrated to obtain the crude product. Dissolve in a single-necked flask with DCM, add active manganese dioxide (55.0 mmol) and stir at room temperature for 10 hours, filter, concentrate the filtrate and spin dry to obtain crude product 3, which is directly used in the next reaction.
[0068] The crude product 3 was added to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com