2-quinolinone-citrinin hybrid dimer compound as well as preparation method and application thereof
A technology of quinolinone and citrinin, which is applied in the field of natural small molecule drugs, can solve the problem that the activity needs to be further developed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Isolation of 2-quinolinone-citrinin hybrid dimer compound
[0040] (1) Strain: Penicillium sp. GGF16-1-2GDMCC No: 61080.
[0041] PDA medium: potato 200g, glucose 20g, sea salt 35g, pure water 1L, pH natural;
[0042] Liquid medium: 1L, maltose 40g, tryptophan 5g, sorbitol 50g, monosodium glutamate 10g, KH2 PO 4 5g, MgSO 4 ·7H 2 O 3g, yeast extract 13g, balance of old seawater, pH 6.5.
[0043] (2) Activation of strains: Use an inoculating loop to pick strains from the strain tube into a culture flask (100mL / 250mL) containing PDA medium, and place it on a constant temperature shaker (165r / min) at 28°C. Cultivated in medium for 2 days to obtain seed liquid.
[0044] (3) Inoculation: suck 1.5 mL of the activated seed culture solution into a 1000 mL culture flask containing 400 mL of liquid medium, and place it in a room at 28° C. for static culture for 60 days, with a total of 150 L of culture.
[0045] (4) Extraction: add 10-20 mL of methanol to each of ...
Embodiment 2
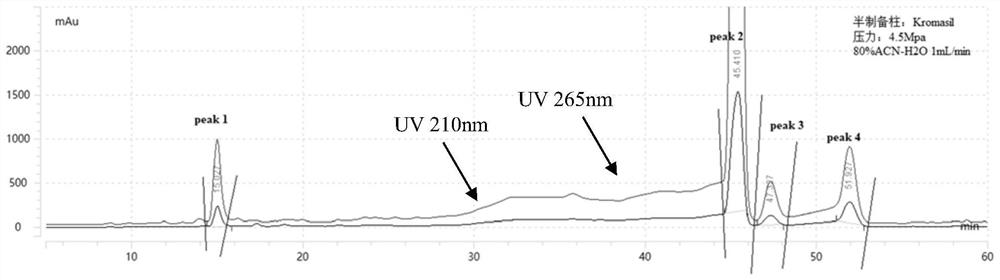
[0048] Example 2: Identification of 2-quinolinone-citrinin hybrid dimer compounds
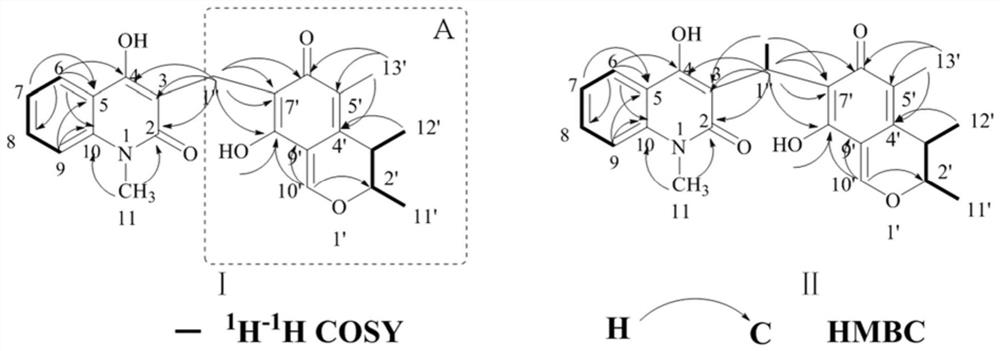
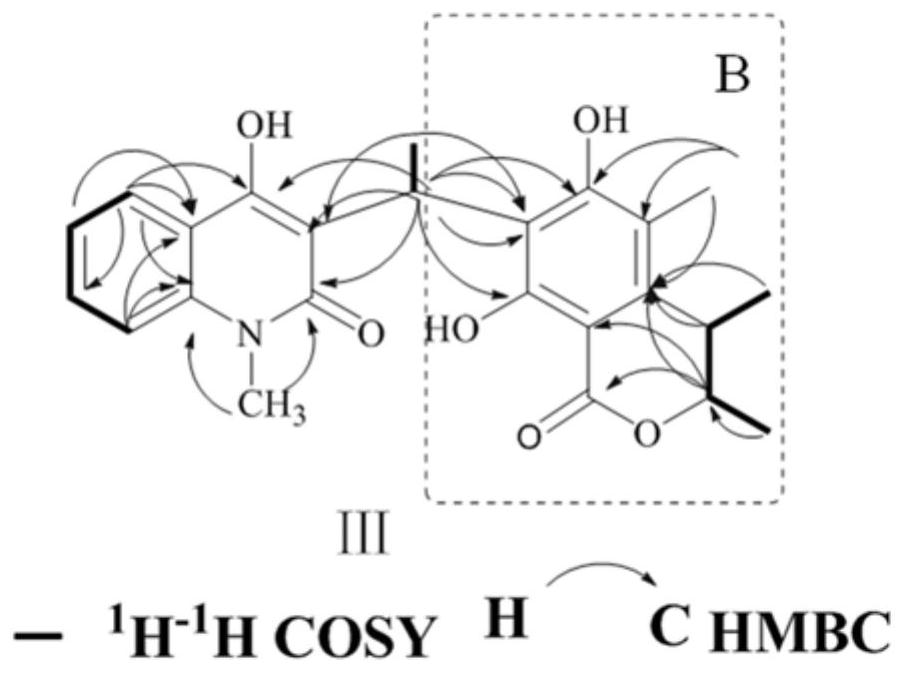
[0049] (1) Compound I, orange-yellow amorphous powder, quasi-molecular ion peak m / z given by positive ion HRESIMS: 394.1647 [M+H] + (Calculated m / z: 394.1649[M+H] + ), determine its molecular formula as C 23 H 23 NO 5 , the desaturation is 13. The maximum absorption wavelengths shown in the ultraviolet spectrum are: 220nm, 286nm and 320nm. Infrared spectrum shows carbonyl (1722cm -1 , 1635cm -1 ) and benzene ring (1608cm -1 , 1519cm -1 , 1494cm -1 and 1457cm -1 ) group.
[0050] 1 H and 13 The results of the C NMR spectrum showed that most of the signals were in pairs and in a 1:1 ratio, probably due to 3 The presence of intramolecular hydrogen bonds in the solvent causes its rotation to be hindered, resulting in two sets of spectra. 1 The H NMR spectrum shows 4 aromatic proton signals δ H 7.27(1H,t,7.6,2.0Hz,H-7), 7.35(1H,d,8.0Hz,H-9), 7.55(1H,t,7.2,1.2Hz,H-8), 8.16(1H, d,8.0...
Embodiment 3
[0071] Example 3: Antibacterial performance test of 2-quinolinone-citrinin hybrid dimer compound:
[0072] Since there is only an antibacterial performance test, the present invention will simultaneously apply for the protection compound and its application, which should be fully disclosed and characterized for a variety of antibacterial properties, so as to expand its application range. It can be considered to adopt a variety of strains including positive bacteria and Negative bacteria were tested.
[0073] (1) Mycelial growth rate method: prepare compound liquids of different concentrations (repeated 3 times for each concentration), pour them into petri dishes for later use, and use the PDA medium plate with sterile water as a control. Take the cultured pathogenic bacteria cake (Staphylococcus epidermidis, Staphylococcus aureus, Methicillin-resistant Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa provided by Guangdong Microorganisms) with a sterile hole p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com