Pig trypsinogen mutant and expression thereof in pichia pastoris
A technique for porcine trypsin and mutant, which is applied in the field of genetic engineering, can solve the problems of toxicity, high toxicity of Escherichia coli, environmental pollution, etc., and achieves the effects of simple fermentation process and purification process, avoiding complicated renaturation operation and broad application prospect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1 Construction and enrichment of recombinant plasmid containing porcine trypsinogen
[0051] The recombinant plasmid was entrusted to Nanjing GenScript Company to synthesize, and CaCl was used after receiving the plasmid powder 2 The chemical transformation method was used to transform into Escherichia coli DH5α competent cells, and the operation steps were as follows:
[0052] Pick a small amount of Escherichia coli with an inoculating loop, inoculate it on the LB medium by streaking, and culture it in a constant temperature incubator at 37°C overnight. , 200r / min shaker culture, use a UV spectrophotometer to detect the OD of the bacterial suspension 600 value when OD 600 When the value is between 0.2-0.5, place on ice to stop growth. Take 1 mL of the above E. coli suspension with a pipette and place it in a centrifuge tube, centrifuge at 4°C and 4000 r / min for 5 min, discard the supernatant; add 100 μL of pre-cooled 0.1 mol / L CaCl to the centrifuge tube res...
Embodiment 2
[0054] Example 2 Construction of porcine trypsin yeast engineering bacteria
[0055] The Pichia pastoris expression plasmid was linearized with restriction enzyme AvrII, and electroporated into Pichia Pastoris X33 competent cells. The specific method is as follows:
[0056] The single colony Pichia X33 on the streak plate was inoculated into 10 mL of YPD liquid medium, and cultured at 30 °C and 250 r / min for 18 to 22 h;
[0057] Detect the OD of the bacterial suspension using a UV spectrophotometer 600 Put 50mL of YPD liquid medium in a 250mL conical flask, calculate the transfer amount according to the following formula, transfer Pichia X33 bacterial liquid, and shake at 30℃ and 250r / min for 18-22h;
[0058]
[0059] Where: OD 始 is the starting OD 600 value; V 1 is the transfer volume; V 2 is medium volume; t is culture time; OD 末 is the OD at the end of the culture 600 value. Example: Measured OD 始 = 16, put 50 mL of YPD liquid medium in a 250 mL conical flask, a...
Embodiment 3
[0067] Example 3 Expression of recombinant porcine trypsin yeast engineering bacteria
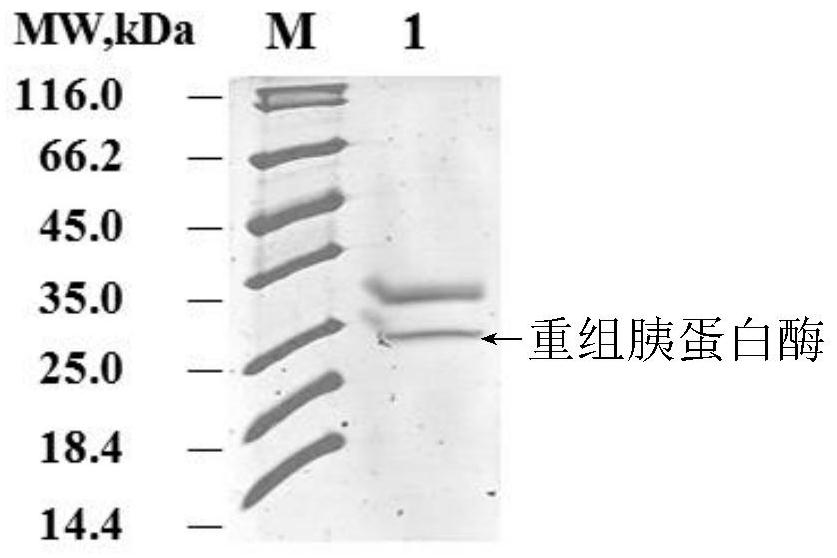
[0068] The above-mentioned recombinant engineered bacteria were inoculated in 10 mL YPD liquid medium according to 2% inoculum, and cultured at 30° C. at 250 r / min. After the cells grew, they were inoculated in 50 mL YPD liquid medium according to 1% inoculum, and 30 Cultivate at 250r / min and express for 48h. Recombinant porcine trypsinogen was secreted and expressed in liquid medium, and the expression of recombinant porcine trypsin was detected by SDS-PAGE. The results are shown in figure 1 (M is Marker, 1: supernatant protein of recombinant trypsin bacterial cell fermentation broth), a relatively obvious expression band appeared around 23kDa in the secreted fluid after expression.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com