Method for preparing 4-O-methyl hematoxylin through separation
A technology of hematoxylin and methyl, applied in the field of separation and preparation of 4-O-methyl hematoxylin, can solve the problems of cumbersome methods, unsuitable for industrial production, and low repeatability, achieve low separation cost, overcome long separation cycle, repeatability, etc. strong effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] A method for separating and preparing 4-O-methylhematoxylin is carried out according to the following steps:
[0036] (1) Extraction of hematoxylin crude extract
[0037] Weigh 1 kg of hematoxylin decoction pieces in the extraction tank, extract 2 times with 5L 75% ethanol under reflux, 2 hours each time, cool and filter, the alcohol extracts are combined and concentrated under reduced pressure under a vacuum rotary evaporator at 50 °C to obtain 97 g of alcohol extract ;
[0038] (2) Preparation of hematoxylin ethyl acetate extract
[0039] After fully dissolving the alcohol extract with water at a ratio of 1:2, extract it with an equal volume (2000 mL) of petroleum ether for 3 times to remove the small polar part in the hematoxylin, remove the phase and then use an equal volume (2000 mL) of ethyl acetate. Ester extraction was performed twice, the ethyl acetate extracts were combined and concentrated under reduced pressure under a vacuum rotary evaporator at 50°C to o...
Embodiment 2
[0047] (1) Extraction of hematoxylin crude extract
[0048] Weigh 1 kg of hematoxylin decoction pieces, and extract 2 times with 5 L of 95% ethanol under reflux for 2 h each time. The extracts are combined and concentrated under reduced pressure under a vacuum rotary evaporator at 50 °C to obtain 60 g of alcohol extract;
[0049] (2) Preparation of hematoxylin ethyl acetate extract
[0050] After fully dissolving the alcohol extract described in the step (1) according to the water with a material-to-liquid ratio of 1:2, first extract it 3 times with an equal volume (2000 mL) of petroleum ether, remove the small polar part in the hematoxylin, remove the phase, and then extract it again. It was extracted twice with equal volume (2000 mL) of ethyl acetate, and the ethyl acetate extract was taken and concentrated under reduced pressure under a vacuum rotary evaporator at 50° C. to obtain 12 g of ethyl acetate extract.
[0051] (3) Preparation of hematoxylin samples
[0052] Weig...
Embodiment 3
[0057] Based on Example 1, the injection concentration in the preparative high performance liquid chromatography was investigated, as shown below, and other steps refer to Example 1.
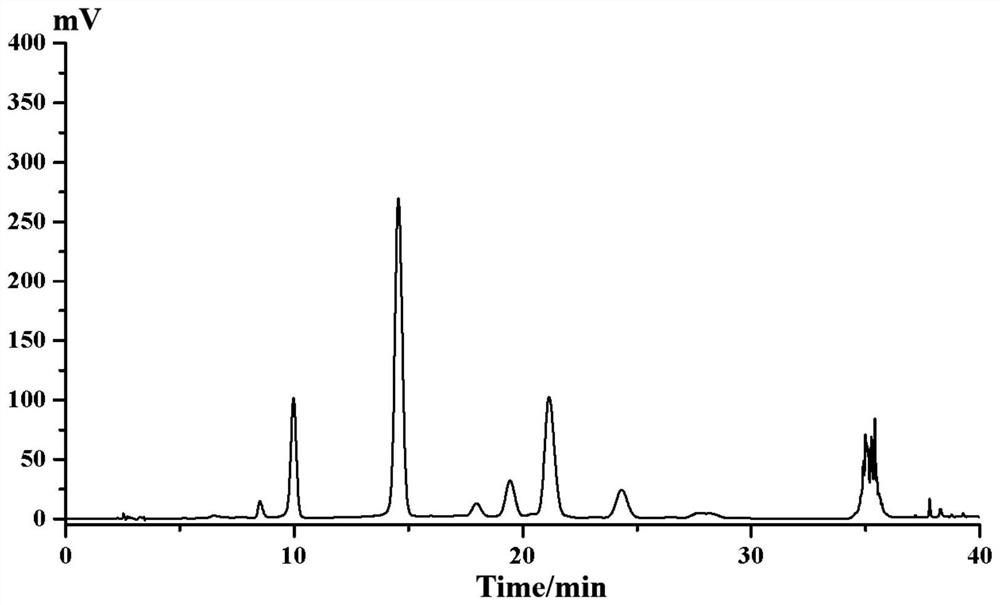
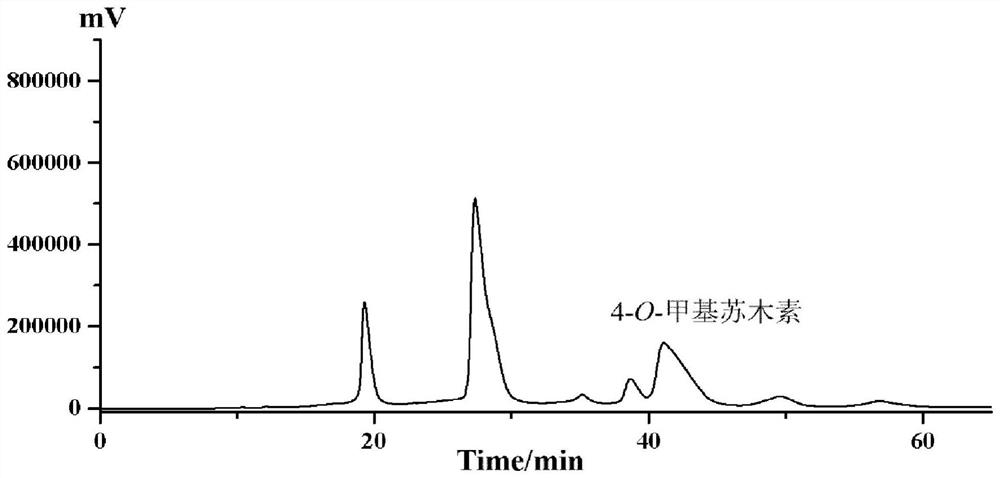
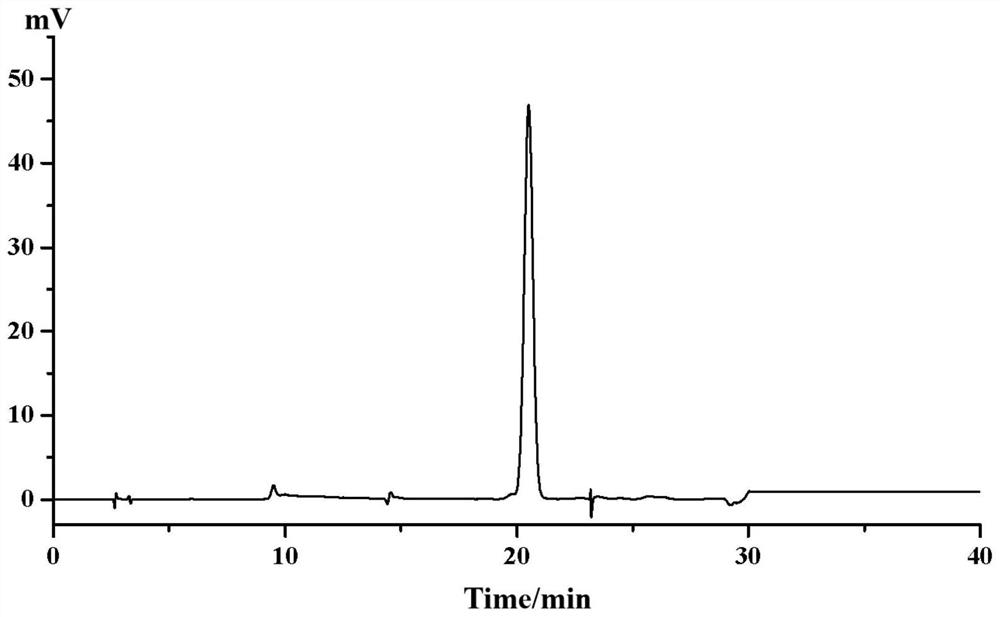
[0058] The hematoxylin sample described in step (3) was dissolved in methanol. A preparative column with octadecylsilane-bonded silica gel as the filler was selected, the model is SHIM-PACK, PRC-ODS (H) (20mm×250mm, 15μm), and acetonitrile (A)-pure water (B) (12: 88, v / v) is the mobile phase, isocratic elution, the injection concentration is 100mg / mL, the injection volume is 1mL, the flow rate is 1.0mL / min, the column temperature is room temperature, the detection wavelength is 285nm, and the liquid chromatogram is collected and prepared Elution fractions with retention times of 40.2-42 min, see Figure 8 . The preparative liquid phase separation effect under this condition is acceptable, but the solvent effect is large due to the large injection volume. The purity of the target fraction was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com