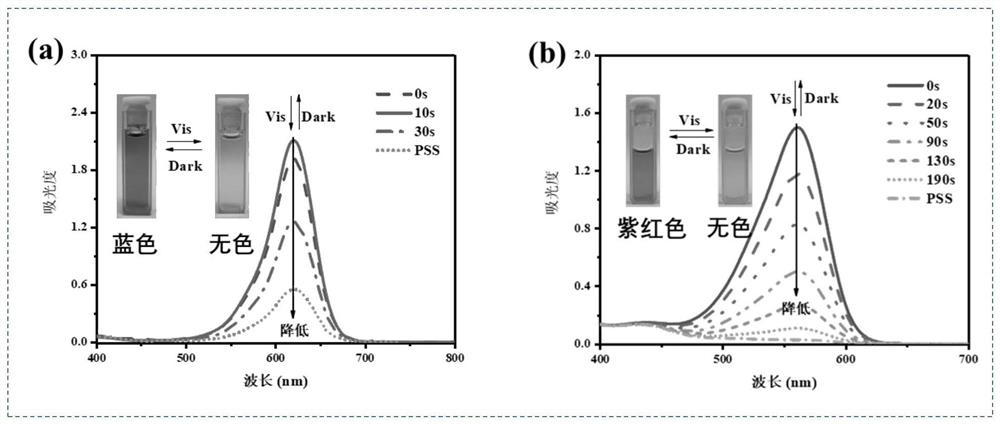
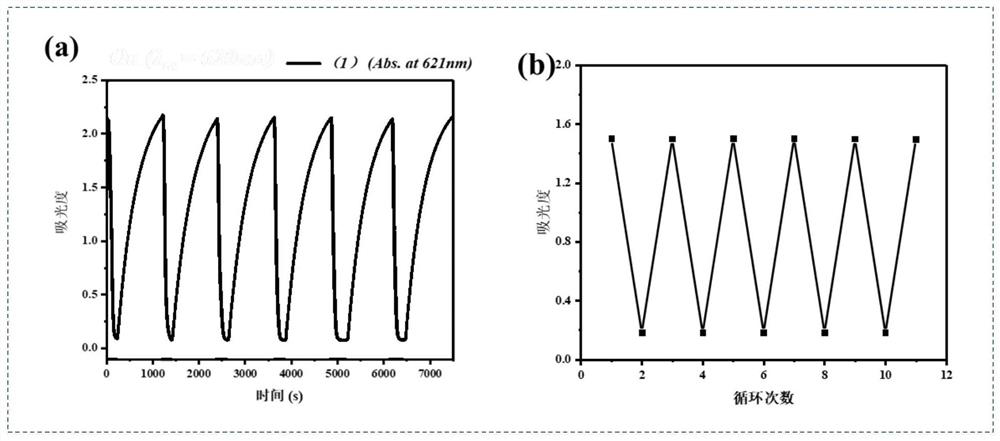
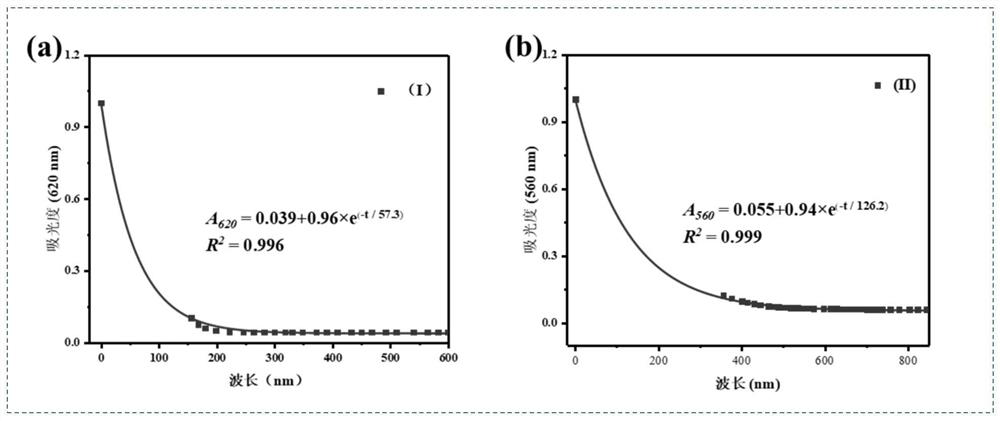
Non-conjugated aniline bridged tetraphenylethylene donor-acceptor Stehausen adduct as well as preparation method and application of non-conjugated aniline bridged tetraphenylethylene donor-acceptor Stehausen adduct
A technology of tetraphenylethylene and conjugated aniline bridges, which is applied in the field of synthesis of new compounds, can solve the problems of insignificant fluorescence intensity, weak fluorescence intensity, and no strong fluorescent groups, etc., and achieve excellent photochromic performance and fast photoresponse rate , The effect of the simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0047] The preferred preparation method of the compound of formula is exemplarily provided below, which should not be construed as a limitation of the present invention by those skilled in the art.
[0048] The synthetic route and preparation method of existing known compounds A1, A2 are as follows:
[0049]
[0050] Preparation of compound A1:
[0051] 1,3-Diethyl-2-thiobarbituric acid (2.00 g, 10.0 mmol) and furfural (830 μL, 10.0 mmol) were added sequentially to 30 mL of H 2 O was stirred at 70°C for 2.5 hours. After the reaction was complete (monitored by TLC), the formed yellow precipitate was collected by vacuum filtration and washed with cold H 2 O wash twice. The resulting solid was dissolved in dichloromethane (75 mL) and washed with 30 mL saturated sodium chloride solution, 30 mL saturated sodium bicarbonate solution, 30 mL H 2 O wash. with MgSO 4 After drying to remove water, the solid was separated by filtration, and the filtrate was collected and concentr...
Embodiment 1
[0057] (1) Preparation of 4-(1,2,2-triphenylvinyl)benzaldehyde
[0058] Take a 250mL three-necked flask, under the protection of nitrogen, 3-bromostyrene (3.340g, 10mmol), 4-formylbenzeneboronic acid (1.500g, 10mmol), potassium carbonate (4.150g, 30mmol), four (tri Phenylphosphine) palladium (0.100g, 0.1mmol) and tetrahydrofuran 80mL were successively added to the there-necked flask, heated to 70° C., refluxed for 12 hours, and the reaction process was monitored by TCL (petroleum ether: ethyl acetate=20:1, v / v). After completion of the reaction, heating was stopped, the mixture was cooled to room temperature, and the solvent was removed by rotary evaporation under reduced pressure. 40 mL of dichloromethane was added, followed by extraction with water (30 mL) and saturated sodium chloride solution (30 mL) three times. The organic layers were combined and dried over anhydrous magnesium sulfate to remove water. After the magnesium sulfate solid was removed by filtration, dich...
Embodiment 2
[0067] (1) Preparation of 4-(1,2,2-triphenylvinyl)benzaldehyde
[0068] Take a 250mL three-necked flask, under the protection of nitrogen, 3-bromostyrene (3.340g, 10mmol), 4-formylbenzeneboronic acid (3.000g, 20mmol), potassium carbonate (4.150g, 30mmol), four (tri Phenylphosphine) palladium (0.200g, 0.2mmol) and tetrahydrofuran 80mL were successively added to the there-necked flask, heated to 80°C, refluxed for 24 hours, and the reaction process was monitored by TCL (petroleum ether: ethyl acetate=20:1, v / v). After completion of the reaction, heating was stopped, the mixture was cooled to room temperature, and the solvent was removed by rotary evaporation under reduced pressure. 40 mL of dichloromethane was added, followed by extraction with water (30 mL) and saturated sodium chloride solution (30 mL) three times. The organic layers were combined and dried over anhydrous magnesium sulfate to remove water. After the magnesium sulfate solid was removed by filtration, dichlo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com