Efficient multiplication culture system of neutrophil and application of efficient multiplication culture system
A technology of neutrophils and neutrophils, applied in the biological field, can solve the problems of neutropenia, high risk of infection in patients, and inability to take immediate effect, achieving remarkable effects and no risk of tumorigenesis Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
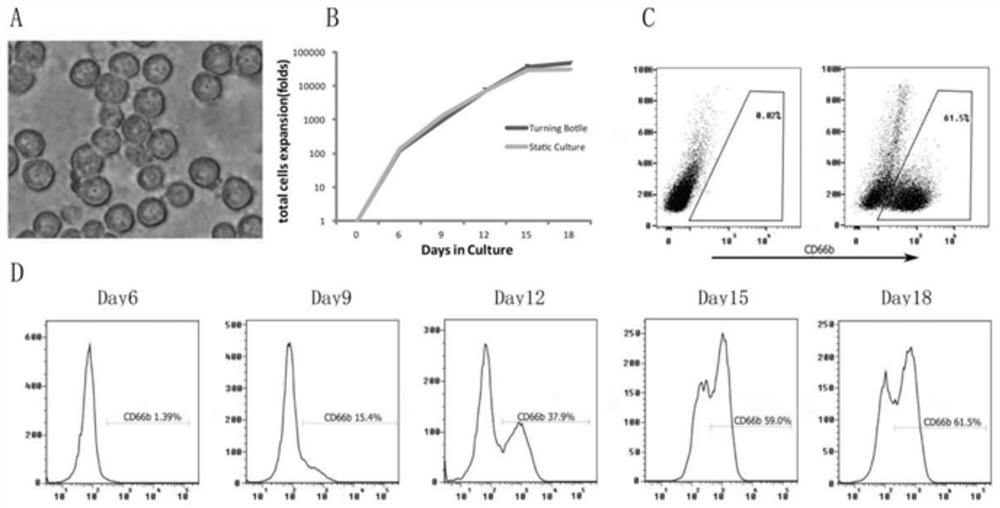
[0039] Example 1: Efficient expansion and culture of human umbilical cord blood-derived neutrophils.
[0040] (1) Extraction of CD34 from umbilical cord blood + cell
[0041] 1) Mix equal volume of fresh umbilical cord blood with sterile PBS (pH 7.2), and calculate the volume ratio of the total volume of umbilical cord blood after mixing to the lymphocyte separation liquid (Ficoll) of 4:3, and put it in a sterile centrifuge tube. First add Ficoll, then slowly add the cord blood to the Ficoll to make it float on the top of the Ficoll;
[0042] 2) After all the addition is complete, carefully take out the centrifuge tube and centrifuge at 2000rpm for 30 minutes. After the centrifugation is completed, take out the mononuclear cell layer and use a 1000μl micropipette to aspirate the white mononuclear cell layer into an empty 50ml centrifuge. spare in the tube;
[0043] 3) Add sterile PBS to make up the volume to 40ml and mix well, take a small amount of solution and count the c...
Embodiment 2
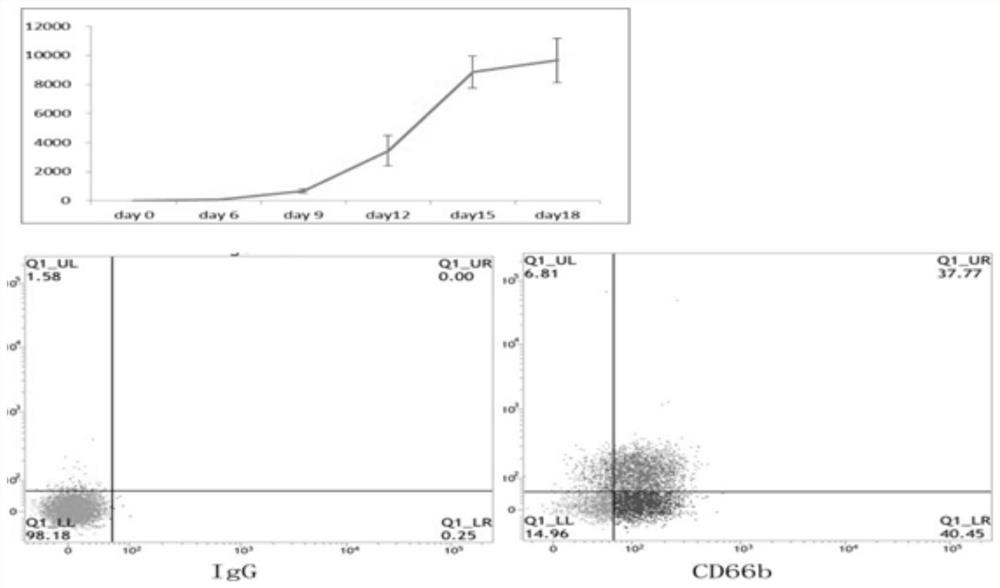
[0063] Example 2 In vitro expansion and differentiation of neutrophils using peripheral blood hematopoietic stem cells.
[0064] The process of mobilizing peripheral blood to isolate hematopoietic stem cells and the above-mentioned use of magnetic bead sorting to sort umbilical cord blood CD34 + The process of hematopoietic stem cells is the same, and the culture method is basically the same. It should be noted that CD34 in peripheral blood during the culture process + The expansion and differentiation speed of hematopoietic stem cells is slightly slower than that of umbilical cord blood hematopoietic stem cells. Counting and changing the medium at each stage should be carried out according to the cell density (the first stage maintains the cell density at 2 × 10). 5 to 1×10 6 cells / mL, the cell seeding density in the second stage was 1.5×10 5 / mL~3×10 5 / mL, then keep no more than 2 × 10 7 / mL). The proportion of neutrophils in the total cells obtained will be much highe...
Embodiment 3
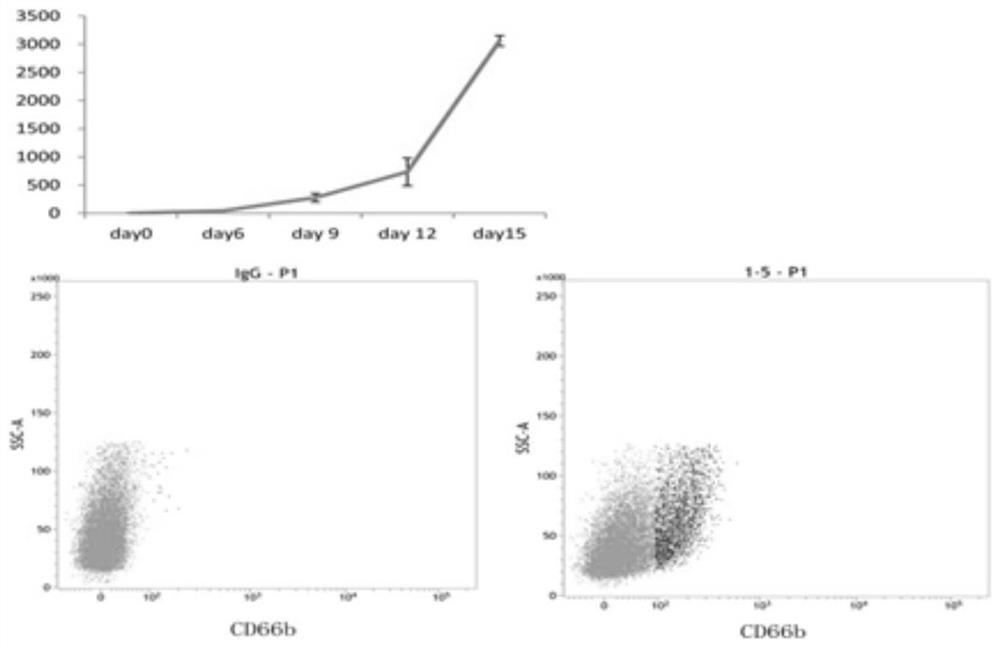
[0066] Example 3: Mobilization of peripheral blood CD34 in non-human primate cynomolgus monkeys + Isolation, in vitro expansion and differentiation of hematopoietic stem cells.
[0067] 1) Cynomolgus monkey peripheral blood hematopoietic stem cell mobilization and culture process: Cynomolgus monkeys were injected subcutaneously with G-CSF (200 μg / kg) and SCF (200 μg / kg) every day for five consecutive days to mobilize bone marrow stem cells in the humerus and femur. into peripheral blood. 15ml of peripheral blood was collected on the 6th and 7th day of injection respectively;
[0068] 2) Use Miltenyi's MACS magnetic bead sorting system to separate CD34 + cells, isolated hematopoietic stem cells (about 2-3 × 10 6 a) The sorting process is the same as the method for human umbilical cord blood hematopoietic stem cells, and the magnetic beads are replaced with non-human primate anti-CD34 magnetic beads;
[0069] 3) Use the culture formula for efficient expansion of hematopoieti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com