Preparation method of gatifloxacin cyclization ester
A technology of gatifloxacin cyclization ester and cyclization reaction, applied in the field of medicine and chemical industry, can solve the problems of inability to recycle, increase the recovery process, low product yield and the like, achieve simplified operation, high atom economy, and by-products high purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
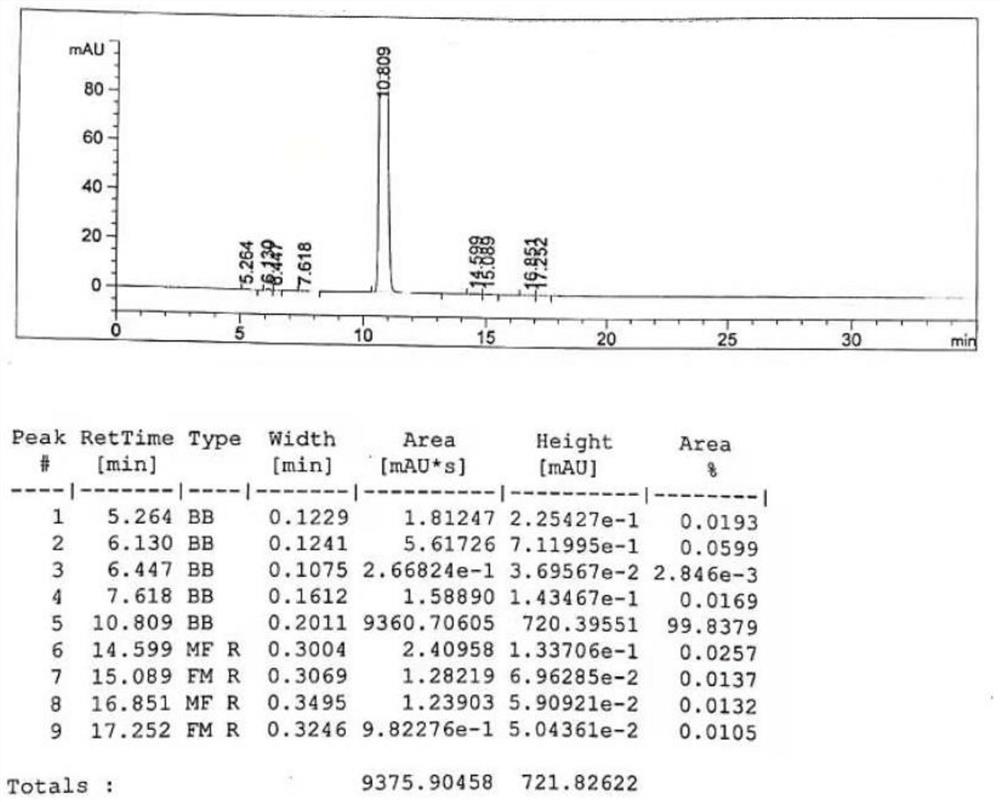
Image
Examples
Embodiment 1
[0042] 1. Coupling reaction
[0043] Put 2,4,5-trifluoro-3-methoxybenzoyl chloride 45g (0.2004mol, 1 times the amount), N,N-dimethylamino ethyl acrylate: 29g (0.2024mol, 1.01 times the amount) into the reaction kettle amount), 150ml of toluene, 0.5g of cuprous iodide, control the vacuum degree to 0.06-0.07Mpa, control the temperature to 35-40℃, keep under reduced pressure for 3h, control the raw material point of the reaction to be ≤0.5%, the reaction is complete, and there are The absorption device absorbs hydrogen chloride with water to obtain by-product hydrochloric acid for the next step of solvent toluene recovery.
[0044] After the reaction was completed, the vacuum was broken with nitrogen, the temperature was lowered to 5-10° C., and 11.44 g (0.2004 mol, 1 times the amount) of cyclopropylamine was added dropwise, and the dropwise addition took 1 h. After the dropwise addition, the temperature was kept at 25-30° C. for 2 hours, and the reaction raw material point was co...
Embodiment 2
[0049] 1. Coupling reaction
[0050] Put 2,4,5-trifluoro-3-methoxybenzoyl chloride 45g (0.2004mol, 1 times the amount), N,N-dimethylamino ethyl acrylate: 29g (0.2024mol, 1.01 times the amount) into the reaction kettle amount), 150ml of toluene, 0.3g of cuprous iodide, the control vacuum degree is 0.06-0.07Mpa, the control temperature is 35-40℃, the vacuum is kept for 3h, the reaction raw material point is controlled to ≤0.5%, the reaction is complete, and there are The absorption device absorbs hydrogen chloride with water to obtain by-product hydrochloric acid for the next step of solvent toluene recovery.
[0051] After the reaction was completed, the vacuum was broken with nitrogen, the temperature was lowered to 5-10° C., and 11.44 g (0.2004 mol, 1 times the amount) of cyclopropylamine was added dropwise, and the dropwise addition took 1 h. After the dropwise addition, the temperature was kept at 25-30° C. for 2 hours, and the reaction raw material point was controlled to...
Embodiment 3
[0056] 1. Coupling reaction
[0057] Put 2,4,5-trifluoro-3-methoxybenzoyl chloride 45g (0.2004mol, 1 times the amount), N,N-dimethylamino ethyl acrylate: 29g (0.2024mol, 1.01 times the amount) into the reaction kettle amount), 150ml of toluene, 0.75g of cuprous iodide, the control vacuum degree is 0.06-0.07Mpa, the control temperature is 35-40℃, the vacuum is kept for 3h, the reaction raw material point is controlled ≤0.5%, the reaction is complete, and there are The absorption device absorbs hydrogen chloride with water to obtain by-product hydrochloric acid for the next step of solvent toluene recovery.
[0058] After the reaction was completed, the vacuum was broken with nitrogen, the temperature was lowered to 5-10° C., and cyclopropylamine 11.44 (0.2004 mol, 1 times the amount) was added dropwise, and the dropwise addition took 1 h. After the dropwise addition, the temperature was kept at 25-30° C. for 2 hours, and the reaction raw material point was controlled to be les...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com