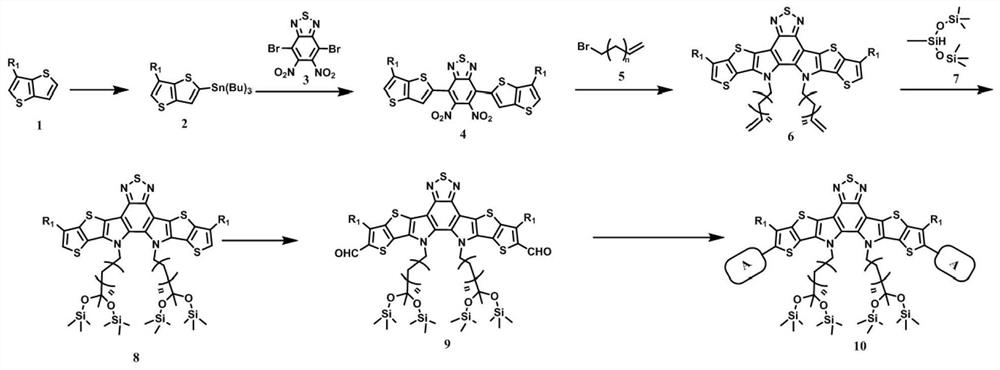
Siloxane group-containing A-DA 'D-A type conjugated organic small molecule as well as preparation method and application thereof
An A-DA, siloxane-based technology, applied in the field of A-DA'D-A type conjugated organic small molecules and their preparation, can solve the problems of hindering π-π interaction, affecting charge transport, etc., to increase the intermolecular Effects of interaction force, high electron mobility, high charge transport performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
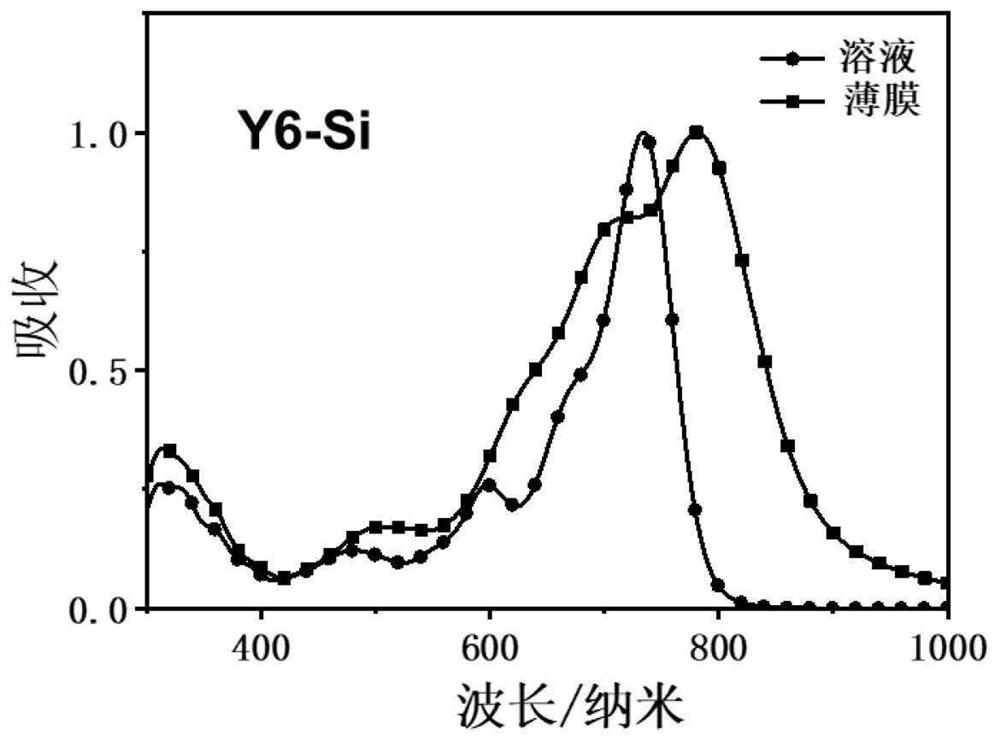
[0058] Example 1 Synthesis of Y6-Si containing siloxane-based organic small molecules
[0059] The present embodiment relates to the preparation of a siloxane-containing A-DA'D-A type conjugated organic small molecule Y6-Si, and the reaction scheme is as follows:
[0060]
[0061] The specific synthesis steps are as follows:
[0062] (1) Synthesis of compound 2
[0063] 3-Undecylthieno[3,2-B]thiophene (27mmol, 7.95g) was dissolved in 100mL of tetrahydrofuran solvent, n-butyllithium (27mmol, 11.34mL) was slowly added under acetone bath, after 2 hours Add tributyltin Bu 3 SnCl (27mmol, 8.78g), then removed the acetone bath, stirred overnight at room temperature, added deionized water to quench the reaction, extracted the organic phase with ethyl acetate, evaporated the solvent, anhydrous MgSO 4 Compound 2 was prepared after drying.
[0064] (2) Synthesis of compound 4
[0065] Under the condition of 110°C, compound 2 (25mmol, 14.6g) prepared in step (1), compound 3 (10mm...
Embodiment 2
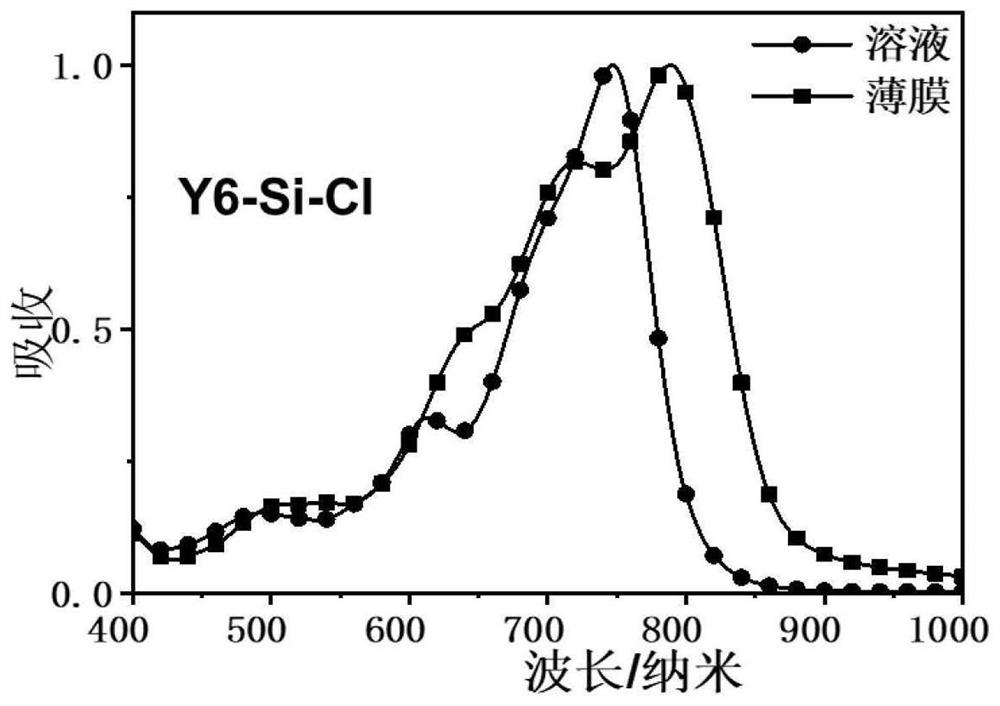
[0082] Example 2 Synthesis of Y6-Si-Cl containing siloxane-based organic small molecules
[0083] The present embodiment relates to the preparation of a siloxane-containing A-DA'D-A type conjugated organic small molecule Y6-Si-Cl, and the reaction scheme is as follows:
[0084]
[0085] where R 1 for -C 11 H 23 .
[0086] The specific synthesis steps are as follows:
[0087] Steps (1) to (5) are the same as in Example 1.
[0088] (6) Synthesis of compound 11:
[0089] Compound 9 (0.50 mmol, 706 mg), 5,6-dichloro-3-(dicyanomethylene)indigo (1.50 mmol, 395 mg), pyridine (0.5 mL) and chloroform (20 mL) were mixed under nitrogen Dissolve in a 100mL two-neck round bottom bottle. The reaction was stirred at 65°C overnight. After cooling to room temperature, the mixture was poured into methanol for sedimentation, and the crude product was obtained after suction filtration. The crude product was purified by silica gel column chromatography, using dichloromethane / petroleum e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com