Azaindole-squarylium cyanine dye as well as synthesis method and application thereof
A technology of azaindole and squaraine, which is applied in its synthesis, azaindole-squarine dyes, biological and medical applications, can solve the problem of affecting the spectral performance of dyes, poor dye stability, and affecting biological Application and other issues, to achieve the effect of good biocompatibility, large molar extinction coefficient, and good survival rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
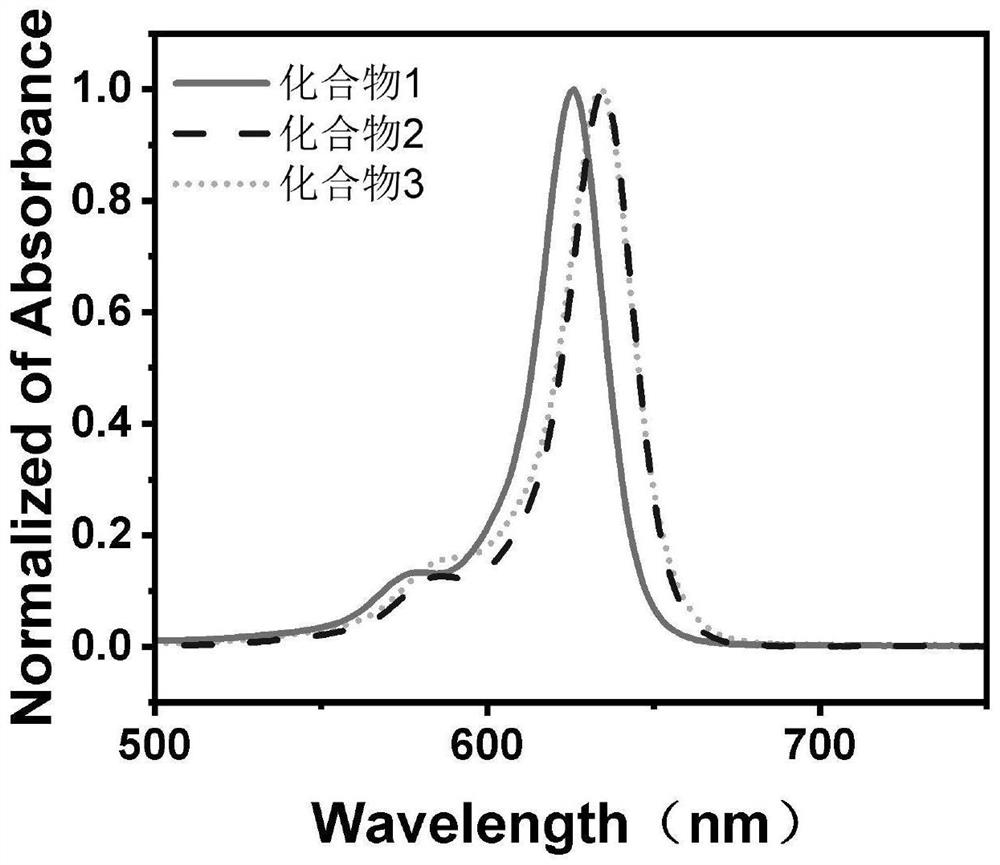
Embodiment 1
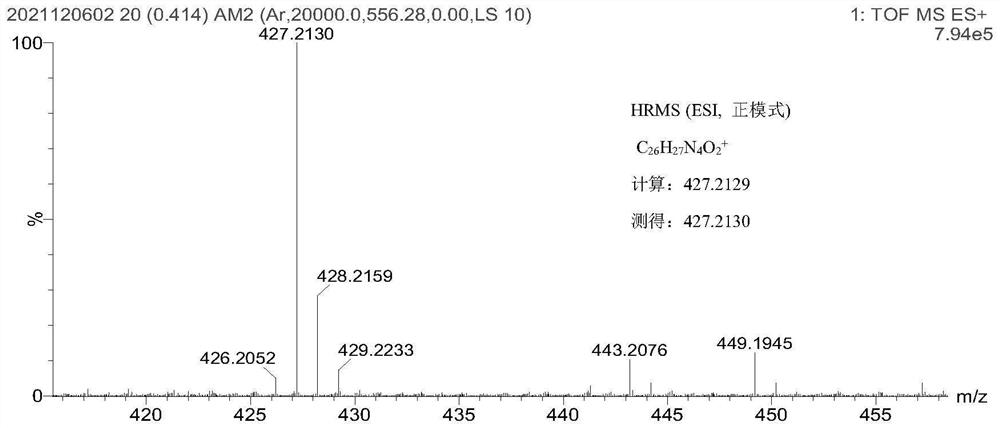
[0052] Example 1 Production of Compound 1
[0053] To 2-hydrazinopyridine (2.182 g, 20 mmol) dissolved in 60 mL of toluene was added 3-methyl-2-butanone (3.445 g, 40 mmol) at room temperature. The mixture was stirred and heated to reflux under nitrogen protection, and the reaction was stopped after 12 h. Cool to room temperature. Most of the toluene was removed, 12 mL of polyphosphoric acid was added to the residue, the reaction was heated and stirred at 140 ° C for 45 min, the mixture was poured into 200 mL of ice water, ammonia water was added dropwise, the pH was adjusted to weakly alkaline, extracted with ethyl acetate Anhydrous Na 2 SO 4 After drying, the solvent was evaporated and purified by silica gel column to obtain compound 1.1 (1.280 g, 8 mmol, Y=40%) as a pale yellow solid.
[0054] The structural formula of compound 1.1 is as follows:
[0055]
[0056] Compound 1.1 (1.00 g, 4 mmol, 1.0 eq) and iodomethane (1.77 g, 8 mmol, 2.0 eq) were added to a 100 mL tw...
Embodiment 2
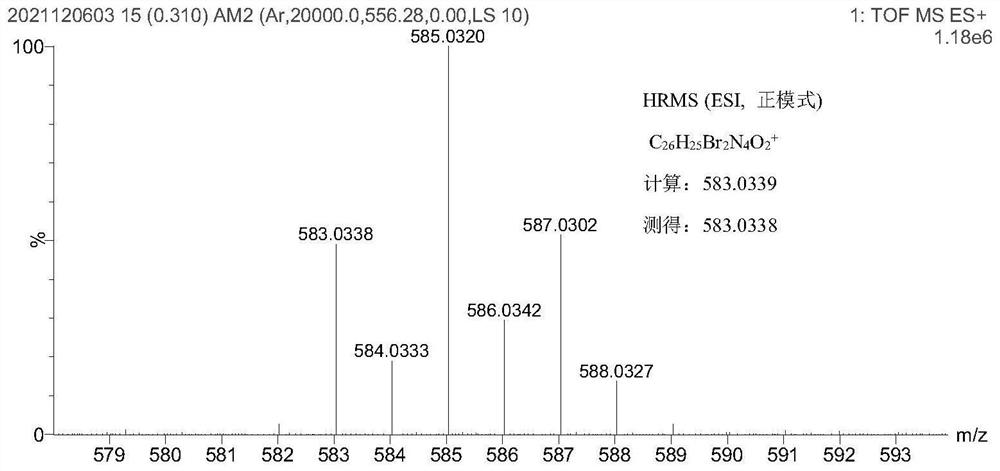
[0062] Example 2 Production of Compound 2
[0063] To 2-hydrazino-4-bromopyridine (1.00 g, 5.32 mmol, 1.0 eq) dissolved in 20 mL of toluene was added 3-methyl-2-butanone (0.92 g, 10.64 mmol, 2.0 eq) at room temperature ). The mixture was stirred and heated to reflux under nitrogen protection, and the reaction was stopped after 12 h. Cool to room temperature. Most of the toluene was removed, 12 mL of polyphosphoric acid was added to the residue, the reaction was heated and stirred at 140 ° C for 45 min, the mixture was poured into 200 mL of ice water, ammonia water was added dropwise, the pH was adjusted to weakly alkaline, extracted with ethyl acetate Na 2 SO 4 After drying, evaporating the solvent and purifying with silica gel column, a pink solid compound 2.1 was obtained (0.760 g, 3.18 mmol, Y=59.8%). The structural formula of compound 2.1 is as follows:
[0064]
[0065] Compound 2.1 (1.00 g, 4.2 mmol, 1.0 eq) and iodomethane (1.19 g, 8.4 mmol, 2.0 eq) were added ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com