Method for selectively oxidizing styrene in water
A styrene oxidation and selective technology, applied in chemical instruments and methods, oxidation preparation of carbonyl compounds, organic chemistry, etc., can solve the problems of toxicity and environmental pollution, safety hazards, high price of organic solvents, etc., and achieve high conversion rate, Short reaction time and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
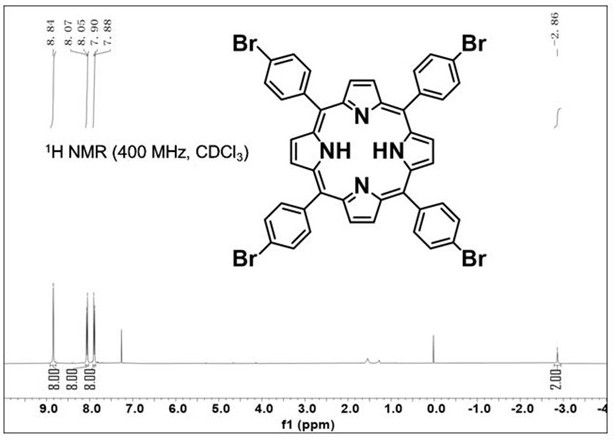
[0061] Pipette pyrrole (2 mL, 1 equiv, 30 mmol) with a pipette, weigh 4-bromobenzaldehyde (5 g, 1 equiv, 30 mmol) with an electronic balance, add it to a 250 mL round-bottomed flask, and weigh 45 in a graduated cylinder. mL propionic acid and 15 mL acetic acid were added to a round-bottomed flask, a magnet was added, heated with an oil bath, stirred at 120 °C for 2 h, cooled to room temperature, evaporated under reduced pressure to remove most of the propionic acid and acetic acid, and methanol After washing three times, 5,10,15,20-tetrakis(4-bromophenyl)porphyrin was obtained after drying. See figure 1 , to prove the nuclear magnetic image of 5, 10, 15, 20-tetrakis (4-bromophenyl) porphyrin obtained in this example.
[0062] Manganese acetate (268 mg, 1 equiv, 1 mmol) and 5, 10, 15, 20-tetrakis(4-bromophenyl) porphyrin (200 mg, 0.33 equiv, 0.33 mmol) were weighed using an electronic balance, and 25 6 mL reaction flask, weigh 6 mL of DMF into the reaction flask, add magnetro...
Embodiment 2
[0065] Styrene (11.5 μL, 0.1 equiv, 0.1 mmol) was added to 1 mL of water, polymanganese porphyrin (5.0 mg, 0.005 equiv, 0.0005 mmol) was added, followed by cumene hydroperoxide (43.4 μL, 0.3 equiv, 0.3 mmol), and the reaction was magnetically stirred for 1 h at 25 °C to obtain the product styrene oxide.
[0066] Among them, the conversion rate of styrene is 42%, and the selectivity of styrene oxide is 84%.
Embodiment 3
[0068] Styrene (11.5 μL, 0.1 equiv, 0.1 mmol) was added to 1 mL of water, polymanganese porphyrin (5.0 mg, 0.005 equiv, 0.0005 mmol) was added, followed by cumene hydroperoxide (43.4 μL, 0.3 equiv, 0.3 mmol), and the reaction was magnetically stirred at 40 °C for 1 h to obtain the product styrene oxide.
[0069] Among them, the conversion rate of styrene is 74%, and the selectivity of styrene oxide is 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com