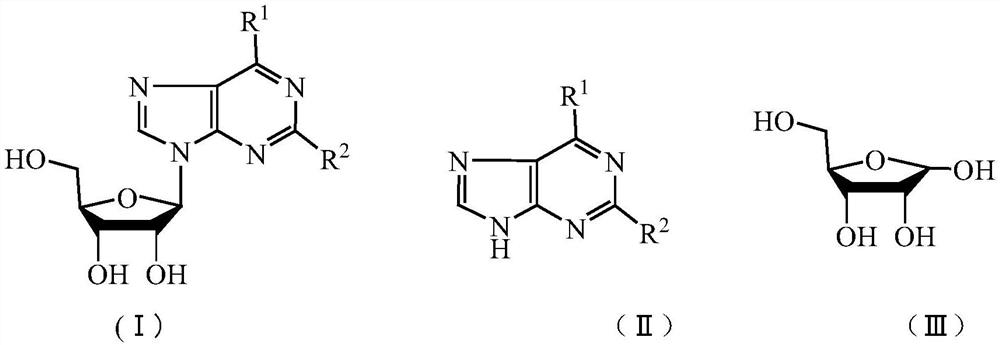
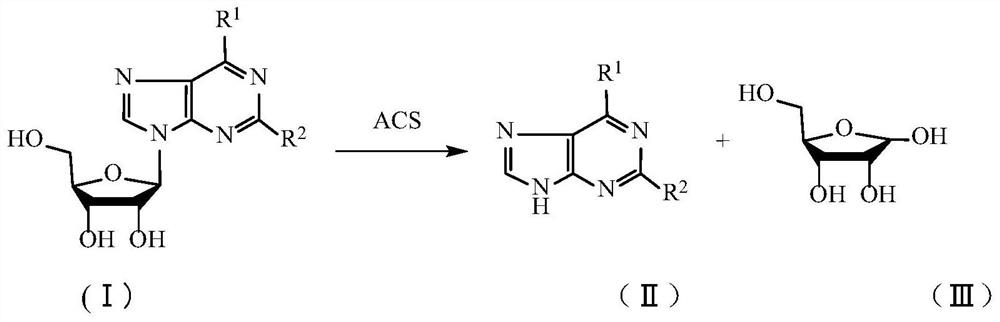
Method for splitting nucleoside compound
A technology for compounds and nucleosides, which is applied in the field of cleaving nucleoside compounds by using acidic calcium sulfate, can solve the problems of complicated separation process, inability to directly obtain D-ribose, long reaction time, etc. The effect of excellent product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The preparation of embodiment 1 acid calcium sulfate (ACS)
[0023] 435mL of concentrated sulfuric acid with a concentration of 98% was added dropwise to a 2-liter flask containing 285mL of deionized water, stirred, cooled to 8°C in an ice bath, and 5g of calcium sulfate was added, and the temperature was controlled between 8 and 12°C. Mix 140 g of calcium hydroxide powder (food grade, with a purity of more than 98%) and 210 g of deionized water, stir, add to the flask in batches, and complete the addition. Continue to keep stirring for 4 hours. Filtration, the filtrate is acid calcium sulfate (ACS).
Embodiment 2A
[0024] Example 2 ACS cleavage of adenosine
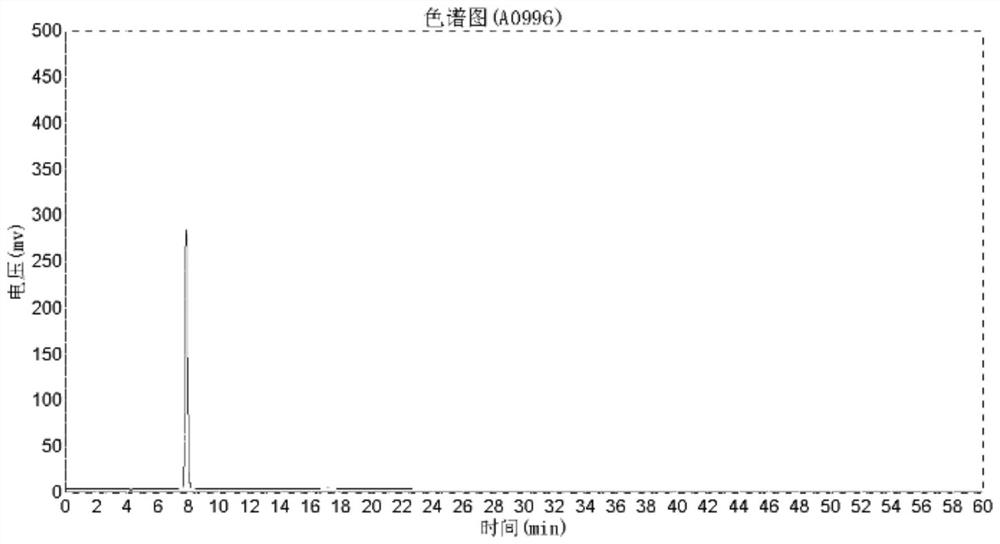
[0025] (1) 10g adenosine was added successively in a 100mL three-necked flask, the acid calcium sulfate prepared by the 20g embodiment 1 method was stirred, and the mixture was heated to 55 ° C and reacted for 5 hours, and sampling was performed with high performance liquid chromatography to detect adenosine conversion rate is 99.5%. After the completion of the reaction, the heating was stopped, cooled to room temperature, and filtered to obtain a filtrate (a brown-yellow aqueous solution containing D-ribose) and a filter cake;
[0026] (2) adding the filter cake of step (1) into a 250mL round-bottomed flask, adding 150mL distilled water, adjusting the pH value of the solution to neutrality (pH is 7) with an aqueous sodium hydroxide solution with a mass concentration of 10%, heating to boiling, and waiting for After the filter cake was completely dissolved in water, the heating was stopped, cooled to room temperature, and left to s...
Embodiment 3A
[0031] Example 3 ACS cleavage of guanosine
[0032] The adenosine in Example 2 was changed to guanosine, and other reaction conditions remained unchanged. 5.07 g of guanine was obtained, the yield was 94.9% (calculated as guanosine), the purity was 99.1%, and the chromatographic detection conditions were the same as those of adenine. 3.2 g of D-ribose solid was obtained. Melting point: 79-81°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com