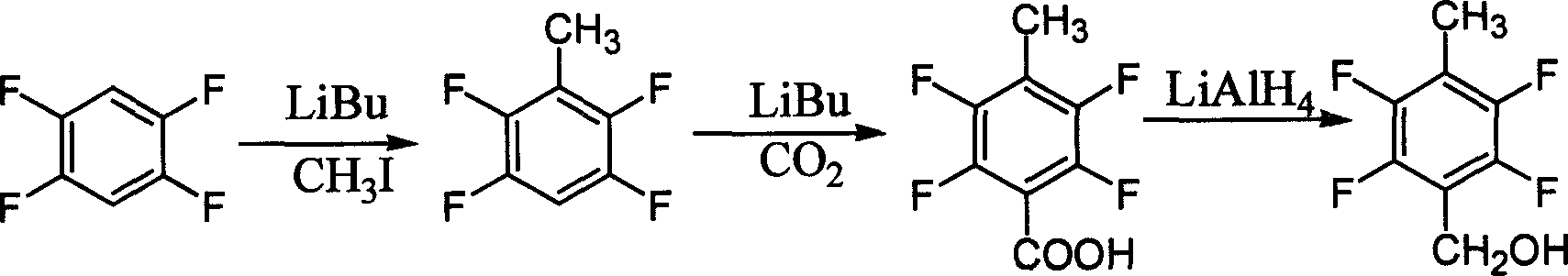Process for preparing 2, 3, 5, 6-tetrafluoro-p-xylyl alcohol
A technology of methyl benzyl alcohol and tetrachloroterephthaloyl chloride is applied in the new preparation field, and can solve the problems of harsh reaction conditions, low total yield of methyl benzyl alcohol, unsuitable for industrial production, etc. The effect of convenient source, simple and reliable process operation and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
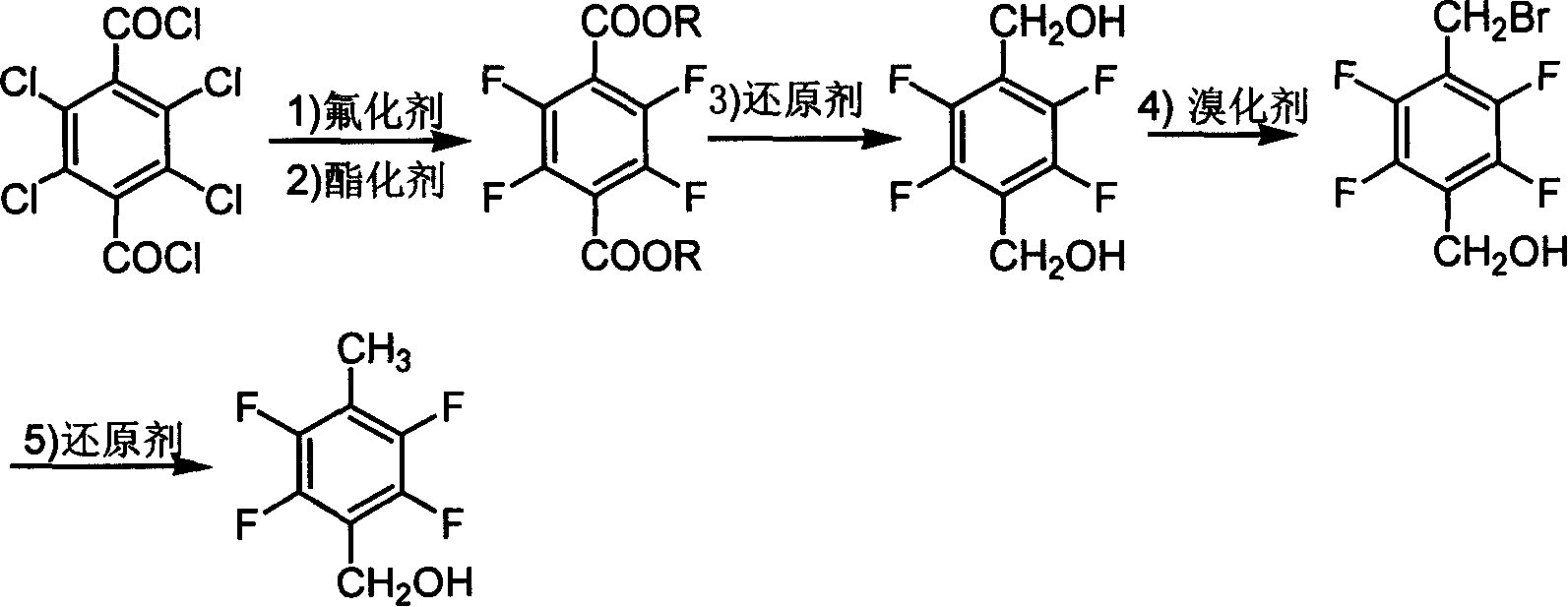
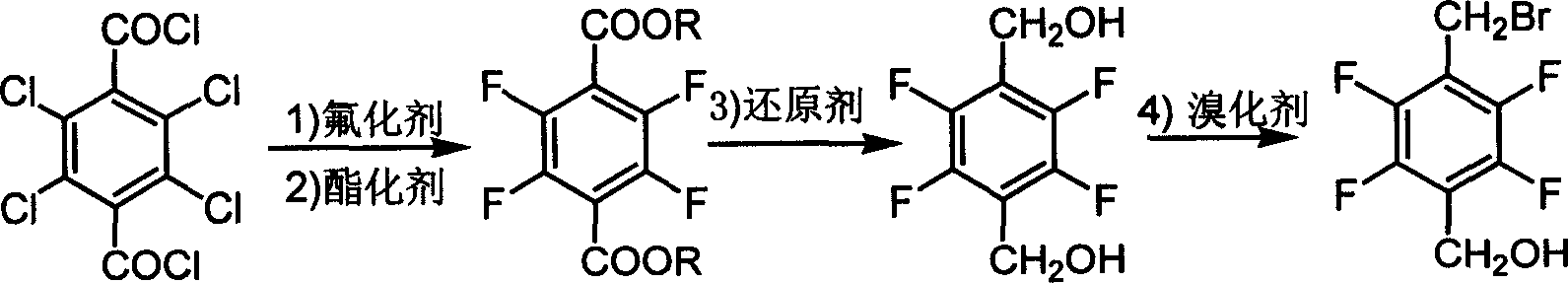
[0019] Preparation of 2,3,5,6-tetrafluoromethyl terephthalate
[0020] Mix 10g of anhydrous potassium fluoride powder, 100mL of sulfolane, 8.75g of tetrachloroterephthaloyl chloride, and 1g of calixarene catalyst into the reactor, heat up to 220°C, react at this temperature for 2 hours, then cool down to 20°C Below ℃, add 100mL of anhydrous methanol, and react at 60℃ for 6h. The esterified product was suction filtered to remove potassium fluoride and inorganic salts. After removing the solvent, the filtrate was added to about 50 mL of water to precipitate a precipitate, filtered with suction, washed with water, and dried to obtain 6.43 g of the product, with a yield of 93.87%. Product melting point: 72°C-74°C, liquid chromatography analysis product purity is 97.01%.
[0021] GC-MS analysis, MS: m / e (relative abundance, %), 266[M, 40], 235[M-OCH 3 , 100], 247 [M-F, 2].
[0022] IR analysis: 1726.4cm -1 , 1485.3cm -1 , 1217.2cm -1 .
example 2
[0024] Preparation of 2,3,5,6-tetrafluoro-tere-phenylene benzyl alcohol
[0025] 1.90g of sodium borohydride was dissolved in 30mL of diglyme, slowly added 5.00g of dimethyl tetrafluoroterephthalate dissolved in 10mL of diglyme was obtained by Example 1, Keep the temperature below 10°C and react for 12h. Extract with dichloromethane, lower the temperature to 0°C, wash with acid and alkali, and recover the solvent to obtain 2.76g of product with a melting point of 122°C to 124°C and a yield of 70.0%. The product has been identified by gas-mass spectrometry.
[0026] MS: m / e (relative abundance, %) 210[M, 100], 209[M-H, 25], 193[M-OH, 2], 189[M-F-2H, 50], 163[M-CH 2 OH-OH+H, 90], 145[M-CH 2 OH-F-OH+2H, 75].
example 3
[0028] Preparation of 2,3,5,6-tetrafluoro-p-bromomethyl benzyl alcohol
[0029] 1 g (4.8 mmol) of 2,3,5,6-tetrafluoro-p-dibenzyl alcohol obtained in Example 2 was added to 5 mL, 10 g (47 mmol) of 48% HBr acid, and 10 mL of benzene were added. The reaction mixture was heated and stirred at 65°C to 70°C for 2h. After the reaction mixture was cooled, the upper toluene layer was separated. After the toluene was dried with anhydrous magnesium sulfate, the toluene was recovered by rotary evaporation to obtain 0.7 g of a light yellow solid, with a yield of 54%. , identified by gas-mass spectrometry, liquid phase analysis of 94% purity.
[0030] The aqueous layer after separation of toluene was extracted with diethyl ether (2×10 mL), and 0.5 g of the raw material was obtained after distilling off the diethyl ether, which was recycled for reuse. The total yield is close to 100% based on raw material consumption.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com