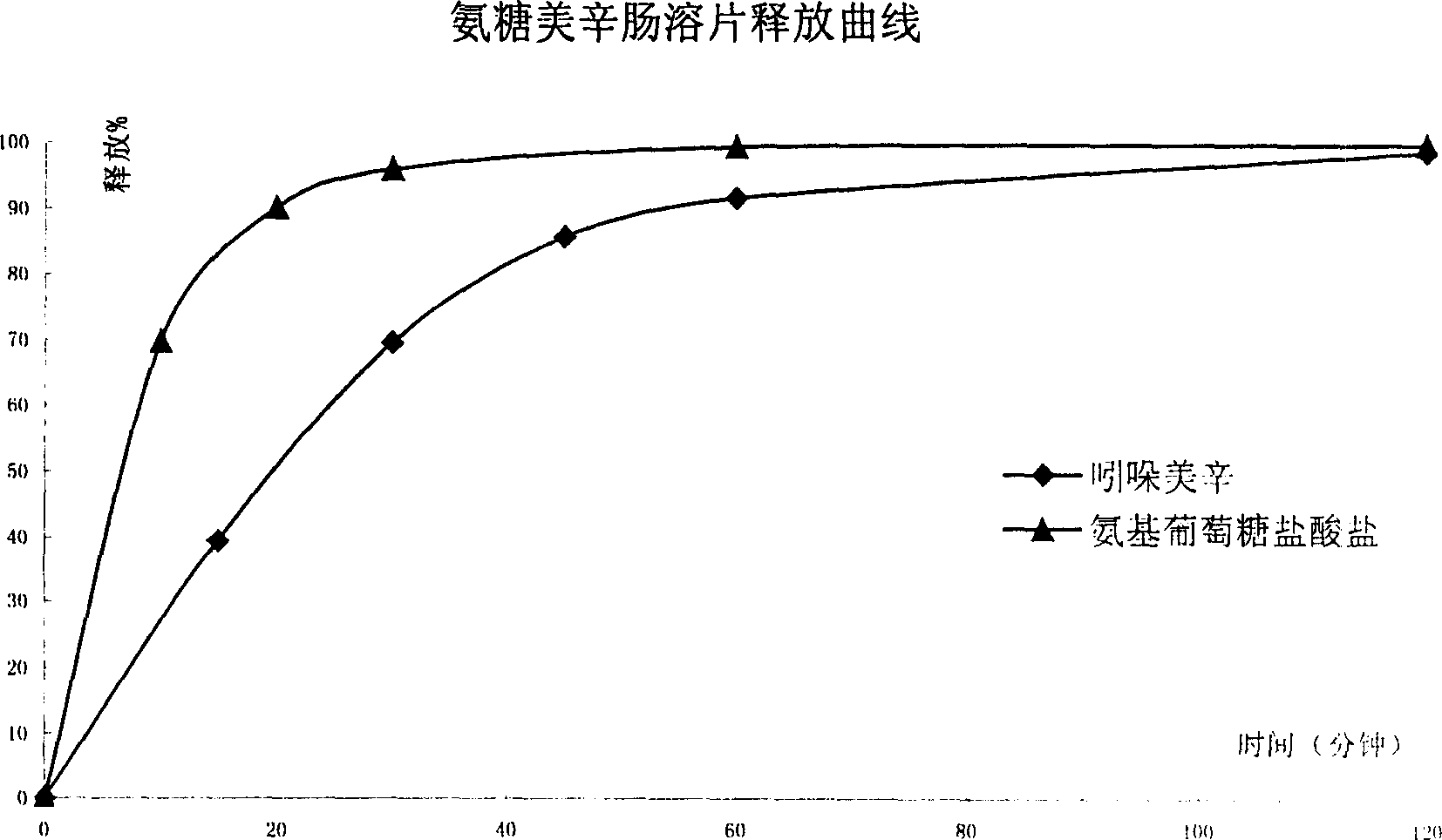
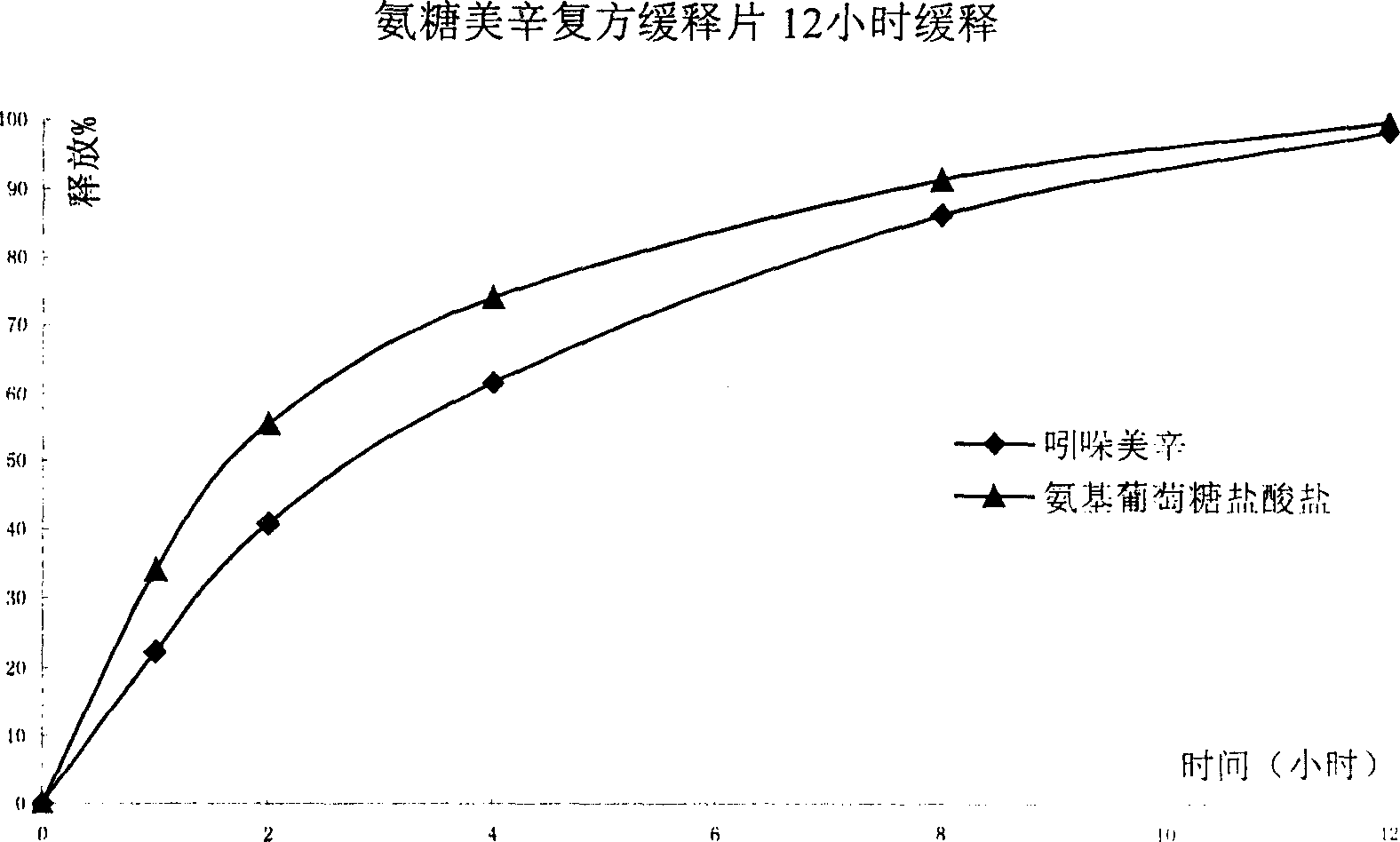
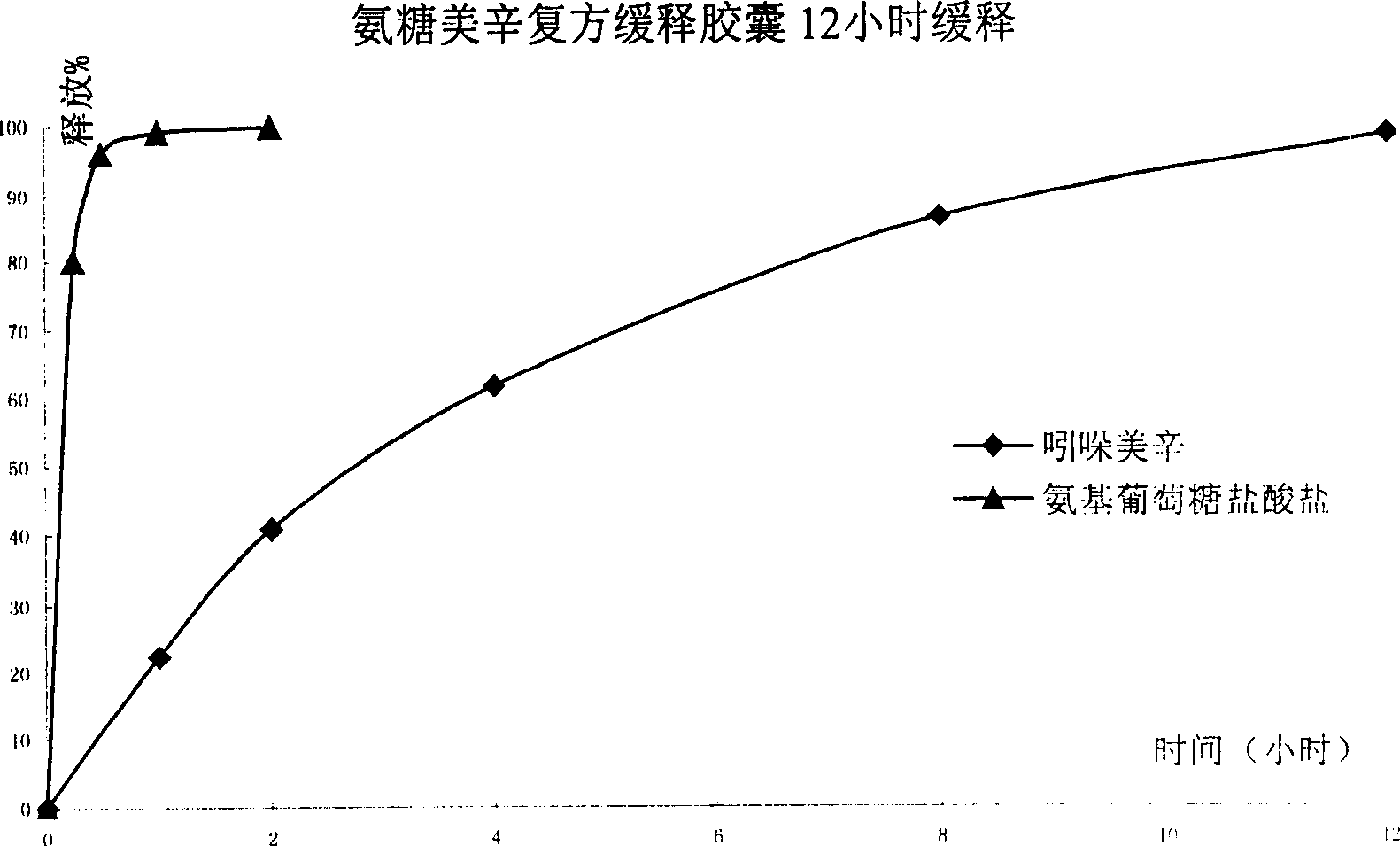
Sustained release formulation of glucosamine salt and preparation
A technology of glucosamine salts and sustained-release preparations, which can be used in anti-inflammatory agents, pharmaceutical formulations, non-central analgesics, etc., and can solve problems such as serious adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The preparation of embodiment 1 matrix type sustained-release tablet
[0036] Indomethacin
Hydroxypropyl methylcellulose (4000 centipoise)
3% hydroxypropyl methylcellulose (50 centipoise) in ethanol
25.0g
75.0g
51.7g
5.3g (calculated as dry matter)
17.5g
0.9g
production
1000 pieces
[0037] Hydroxypropyl methylcellulose is a polymer of hydrophilic skeleton material, which swells with water or digestive juice to form a gel barrier to control the release rate of indomethacin and glucosamine hydrochloride and achieve the purpose of sustained release.
[0038] Preparation:
[0039] 1. Both glucosamine and indomethacin are made into sustained-release preparations: the amount of glucosamine hydrochloride and indomethacin, hydroxypropyl methylcellulose, microcrystalline fiber Mix well, add 3% hydroxypropyl methylcellulose e...
Embodiment 2
[0042] The preparation of embodiment 2 matrix type sustained-release tablets
[0043] Glucosamine hydrochloride
Indomethacin
Ethylcellulose (50 centipoise)
2% ethylcellulose (50 centipoise) in absolute ethanol
225.0g
75.0g
42.8g
123.0g
20.0g (calculated as dry matter)
64.0g
production
1000 pieces
[0044] Stearic acid is a bioerodible material, and ethyl cellulose is a non-erodible framework material. The two are used in combination to control the release of indomethacin and glucosamine hydrochloride in the preparation.
[0045] Preparation:
[0046] 1. Both glucosamine and indomethacin are made into sustained-release preparations: mix the indomethacin, glucosamine hydrochloride, ethyl cellulose and stearic acid in the amount of the above-mentioned formula of this embodiment, and then add Ethyl cellulose solution in absolute ethanol is mixed well, granulated thro...
Embodiment 3
[0049] The preparation of embodiment 3 film-controlled sustained-release capsules
[0050] Glucosamine Hydrochloride
Indomethacin
2% hydroxypropyl methylcellulose (50 centipoise) in water
Trimethylammonium ethyl acrylate-methacrylate copolymer
(Eudragit RS100)
Polyvinylpyrrolidone K30 ethanol solution
(K30 is the molecular weight index of the polymer obtained by measuring the viscosity of the polymer)
150.0g
50.0g
6.3g (calculated as dry matter) methyl
50.0g
43.8g
12.5g
production
1000 capsules
[0051] Using Eudragit RS100 as the coating material, a small amount of polyvinylpyrrolidone ethanol solution (PVPk30) was added to the coating solution as a porogen. After the porogen dissolved in the digestive juice, micropores were formed on the coating. Slow and smooth release in the hole.
[0052] Preparation:
[0053] 1. Both glucosamine and indomethacin a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com