Cobaltosic oxide preparation method for lithium ion battery
A technology for tricobalt tetroxide and lithium ion batteries, which is applied in the directions of cobalt oxide/cobalt hydroxide, battery electrodes, circuits, etc., can solve problems such as no involvement, and achieve high bulk density, improved electrical performance and service life, and high conversion rate. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
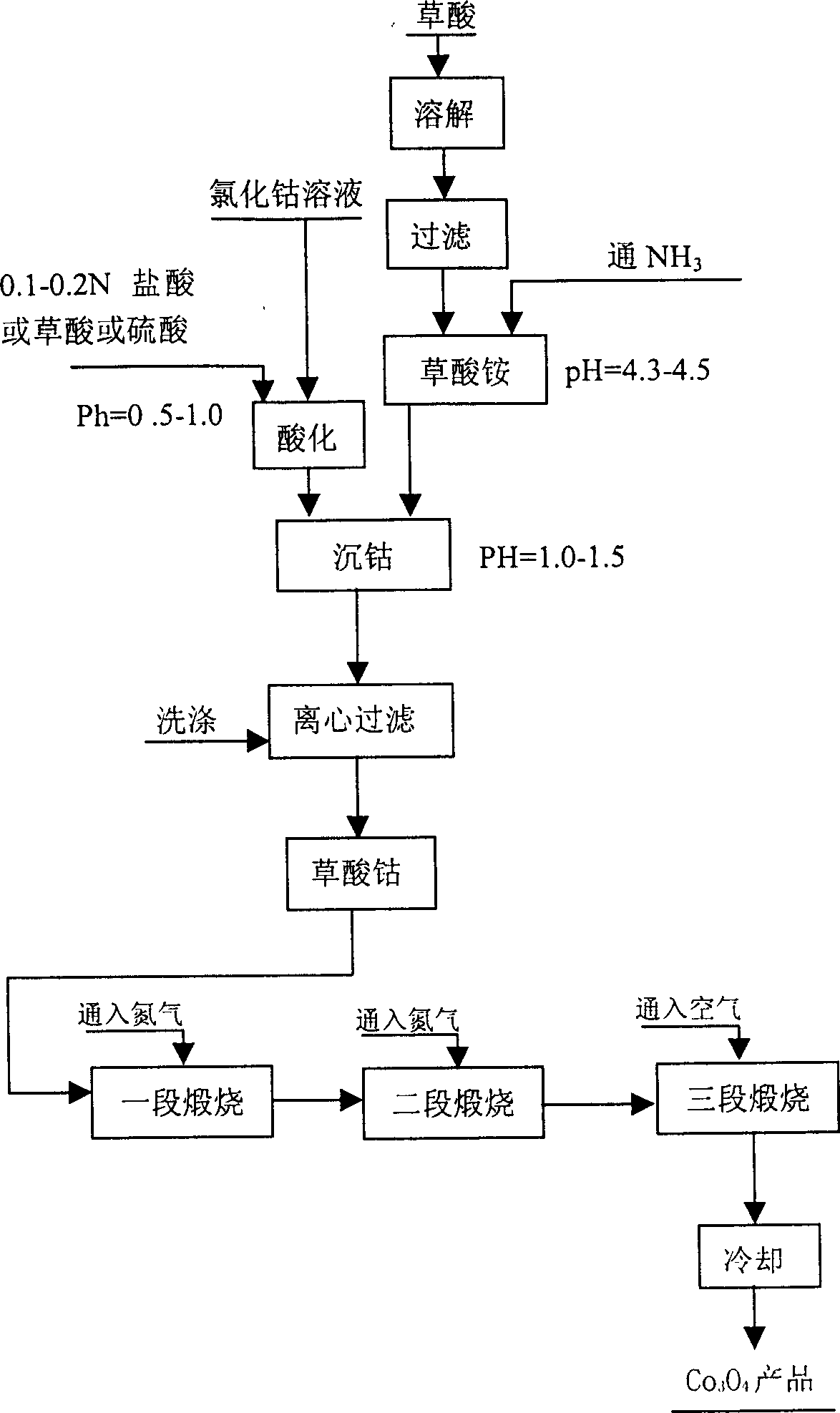
[0048] use as figure 1 The processing method shown prepares cobalt tetroxide, and its steps are:
[0049] 1. Preparation of spherical cobalt oxalate powder:
[0050] a. Prepare an aqueous solution of cobalt chloride, mix aqua regia (HCl:HNO) 3 =3:1) heated to 80-90 ℃, the electrolyzed cobalt sheet was dissolved therein to form a cobalt chloride solution with a concentration of 100 g / L, and oxalic acid was added for acidification, so that the pH of the cobalt chloride aqueous solution was 0.6- 0.8;
[0051] b. Preparation of ammonium oxalate aqueous solution: pass NH into oxalic acid 3 , so that the concentration is 90 g / L, and the pH of the ammonium oxalate aqueous solution is 4.3-4.5; in the ammonium oxalate solution, a dispersant is added, which is polyethylene glycol (molecular weight is 4000), and the addition amount of the dispersant is Add 6.25 grams per 10 liters of aqueous ammonium oxalate.
[0052] c. Add the ammonium oxalate aqueous solution prepared in step b i...
Embodiment 2
[0068] 1. Preparation of spherical cobalt carbonate powder:
[0069] a. Prepare an aqueous cobalt chloride solution with a concentration of 60 g / L, and add sulfuric acid to make the pH of the cobalt salt aqueous solution 0.5-0.6;
[0070] b. Prepare an aqueous ammonium bicarbonate solution with a concentration of 70 g / L, and introduce ammonia to make the pH of the aqueous ammonium bicarbonate solution 7-9; add a dispersant to the ammonium bicarbonate solution, which is maleic butadiene and acrylic acid The ratio of the mixture is: 1:1, and the addition amount of the dispersant is 7 grams per 10 liters of ammonium bicarbonate aqueous solution.
[0071] c. The ammonium bicarbonate aqueous solution is sprayed into the cobalt salt aqueous solution until the pH of the mixed solution reaches 7.5-8.5, and the above pH is maintained during the reaction process. The method for maintaining the pH unchanged is to make the cobalt salt solution. The addition amount of ammonium bicarbonate...
Embodiment 3
[0084] 1. Preparation of spherical cobalt hydroxide powder:
[0085] a. Prepare an aqueous cobalt chloride solution with a concentration of 120 g / L, and add hydrochloric acid to make the pH of the aqueous solution 0.9-1.1;
[0086] b. Preparation of ammonia-containing NaOH solution, its concentration is 20%, and the ammonia water whose specific gravity d is 95% is introduced, and the volume ratio of NaOH (20%) and ammonia water (specific gravity=0.95) is 8:1; The dispersant is a mixture of butadiene and acrylic acid, the ratio of which is 1:1, and the addition amount of the dispersant is 7 grams per 10 liters of NaOH aqueous solution.
[0087] c. The NaOH solution obtained in step b is sprayed into the cobalt raw material aqueous solution, and the pH is maintained at 10-11, so that the reaction is carried out at a stable pH. The way to maintain pH is to stabilize the added amount of cobalt chloride and adjust the added amount of NaON solution at any time. The reaction temper...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Bulk density | aaaaa | aaaaa |
| Bulk density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com