Sol-gel in situ and self assembling process for synthesizing compound photocatalystic material
A technology of composite photocatalysis and synthesis method, which is applied in the field of sol-gel in-situ and self-assembly synthesis of composite photocatalytic materials, which can solve the problems of affecting photocatalytic efficiency, difficulty in dissolving iron phthalocyanine, and reducing photocatalytic efficiency. , to achieve the effects of improving photocatalytic efficiency, enhancing strong interfacial interaction, and good photostability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
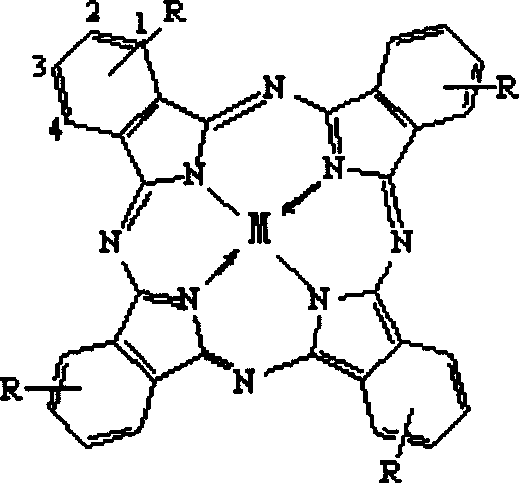
[0050] From figure 1 It can be seen that the metal phthalocyanine complex (Phthalocyanine) has a planar macrocyclic molecule with a highly conjugated π-electron system.
[0051] (1) Weigh a certain amount of cobalt chloride (CoCl 2 ) (analytically pure), dissolved in hydrochloric acid to make solution one.
[0052] (2) Weigh a certain amount of phthalonitrile (analytical pure), and dissolve it in absolute ethanol, wherein the molar ratio of phthalonitrile and metal chloride salt is 4:1, and make solution 2.
[0053] (3) Slowly add solution 2 into solution 1 to form a mixed solution, and stir for 30 minutes.
[0054] (4) An appropriate amount of butyl titanate solution (analytically pure) is added to the above mixed solution, wherein the nominal molar ratio of the metal ion in the metal salt to the metal titanium in the butyl titanate is 1.0 mole percent).
[0055] (5) Hydrochloric acid (HCl) was added dropwise until the pH value of the above (4) solution was about 2.0.
[...
Embodiment 2
[0065] (1) Weigh a certain amount of copper chloride (CuCl 2 ) (analytically pure), dissolved in a certain amount of absolute ethanol to make Solution 1.
[0066] (2) Weigh a certain amount of phthalonitrile (analytical pure), and dissolve it in absolute ethanol, wherein the molar ratio of phthalonitrile and metal chloride salt is 4:1, and make solution 2.
[0067] (3) Slowly add solution 2 into solution 1 to form a mixed solution, and stir for 30 minutes.
[0068] (4) An appropriate amount of butyl titanate solution (analytically pure) is added to the above mixed solution, wherein the nominal molar ratio of the metal ion in the metal salt to the metal titanium in the butyl titanate is 1.0 mole percent).
[0069] (5) Hydrochloric acid (HCl) was added dropwise until the pH value of the above (4) solution was about 2.0.
[0070] (6) Stir for about 100 hours at room temperature until gel and xerogel are formed, and after grinding for 2 hours, the precursor of the nanocomposite ...
Embodiment 3
[0079] (1) Weigh a certain amount of cuprous chloride (CuCl) (analytically pure), dissolve it in hydrochloric acid, and make solution one.
[0080] (2) Weigh a certain amount of phthalonitrile (analytical pure), and dissolve it in absolute ethanol, wherein the molar ratio of phthalonitrile and metal chloride salt is 4:1, and make solution 2.
[0081] (3) Slowly add solution 2 into solution 1 to form a mixed solution, and stir for 30 minutes.
[0082] (4) An appropriate amount of butyl titanate solution (analytically pure) is added to the above mixed solution, wherein the nominal molar ratio of the metal ion in the metal salt to the metal titanium in the butyl titanate is 1.0 mole percent).
[0083] (5) Hydrochloric acid (HCl) was added dropwise until the pH value of the above (4) solution was about 2.0.
[0084] (6) Stir for about 100 hours at room temperature until gel and xerogel are formed, and after grinding for 2 hours, the precursor of the nanocomposite photocatalyst is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com