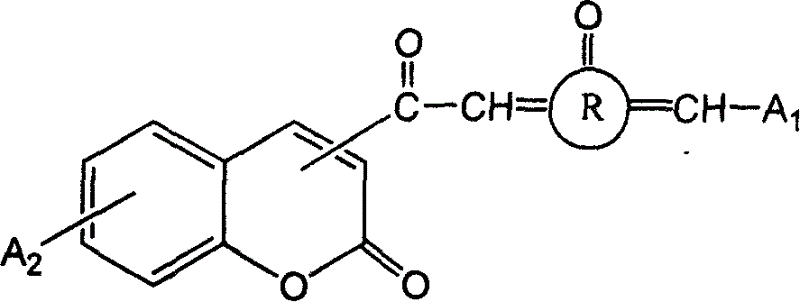
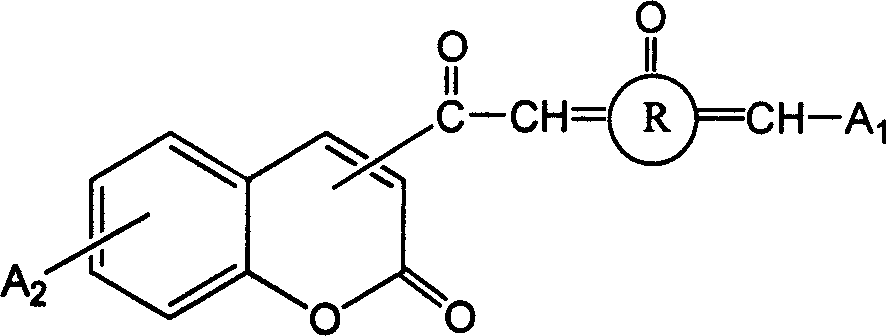
3- or 4- carbonyl substituted coumarin connected with naphthenones and its synthesis method and use
A technology of cycloalkane ketone and coumarin ketone, which is applied in the field of visible light sensitizing dyes, can solve the problems of wide absorption band coverage and the like, and achieve the effect of wide absorption band
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0112] Synthesis of Ketocoumarin Substituted by Carbonyl at 3 or 4 Position Linked by Cyclopentanone (CP-KETOCOU)
[0113] (1) 40 millimoles of cyclopentanone and 10 millimoles of p-dimethylaminobenzaldehyde are placed in a flask, add 7 milliliters of dehydrated alcohol and 10 milliliters of water, drop 0.5 milliliters of hexahydropyridine, and After reacting at 25°C for 5 hours, the orange precipitate was filtered and separated by column chromatography with 0.2wt% ethanol / chloroform to obtain half-p-dimethylaminobenzylidene cyclopentanone dye with a yield of 85%.
[0114] (2) (synthetic method refers to literature "Journal of Medicinal Chemistry" 1983 volume 31 page 3014, ChemPharm Bull, 1983,31,3014) with 10 mmoles of 4-acetyl-5-pyrrolylcoumarin, 12 mmoles Put selenous acid in a flask, add 50 ml of xylene to heat and dissolve, reflux for 10 hours, heat filter to remove gray-black selenium, leave the filtrate to cool and precipitate red needle-like crystals, and filter to obt...
Embodiment 2
[0117] Synthesis of Ketocoumarin (CHE-KETOCOU) Substituted by 3 or 4 Carbonyl Linked by Cyclohexanone
[0118] (1) Put 30 millimoles of cyclohexanone and 10 millimoles of acetaldehyde into a flask, add 20 milliliters of absolute ethanol and 5 milliliters of water, drop 2 milliliters of 10 wt % NaOH aqueous solution, and react at 60 ° C for 24 hours , filtered the orange precipitate, and separated by column chromatography with 0.2wt% ethanol / chloroform to obtain half 2-ethylcyclohexanone dye with a yield of 75%.
[0119] (2) (for the synthesis method, refer to the literature "Acta Medicinal Chemistry" 1983, Volume 31, page 3014, ChemPharm Bull, 1983, 31, 3014) with 10 mmoles of 3-acetyl-7-methoxycoumarin, 15 mmoles Put molar selenous acid in a flask, add 60 milliliters of ethanol to heat and dissolve, reflux for 15 hours, heat filter to remove gray-black selenium, leave the filtrate to cool and precipitate yellow needle-like crystals, and filter to obtain pure 3-ketoaldehyde B...
Embodiment 3
[0122] Synthesis of Ketocoumarin (CHP-KETOCOU) Substituted by 3 or 4 Carbonyl Linked by Cycloheptanone
[0123] (1) Put 20 millimoles of cycloheptanone and 10 millimoles of benzaldehyde into a flask, add 20 milliliters of absolute ethanol and 2 milliliters of water, add dropwise 3 milliliters of 10 wt % KOH aqueous solution, and react at 65 ° C for 36 hours , filtered the orange precipitate, and separated by column chromatography with 0.2wt% ethanol / chloroform to obtain a hemibenzylidene cycloheptanone dye with a yield of 65%.
[0124] (2) (synthetic method refers to literature "Acta Medicinal Chemistry" 1983 volume 31 page 3014, ChemPharm Bull, 1983,31,3014) 10 mmoles of 3-methyl-6-phenylcoumarin, 18 mmoles Put selenous acid in a flask, add 70 ml of 1,4-dioxane and heat to dissolve, reflux for 24 hours, heat filter to remove gray-black selenium, let the filtrate stand and cool to precipitate red crystals, and filter to obtain pure product 3-ketoaldehyde-6-phenylcoumarin, yie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com