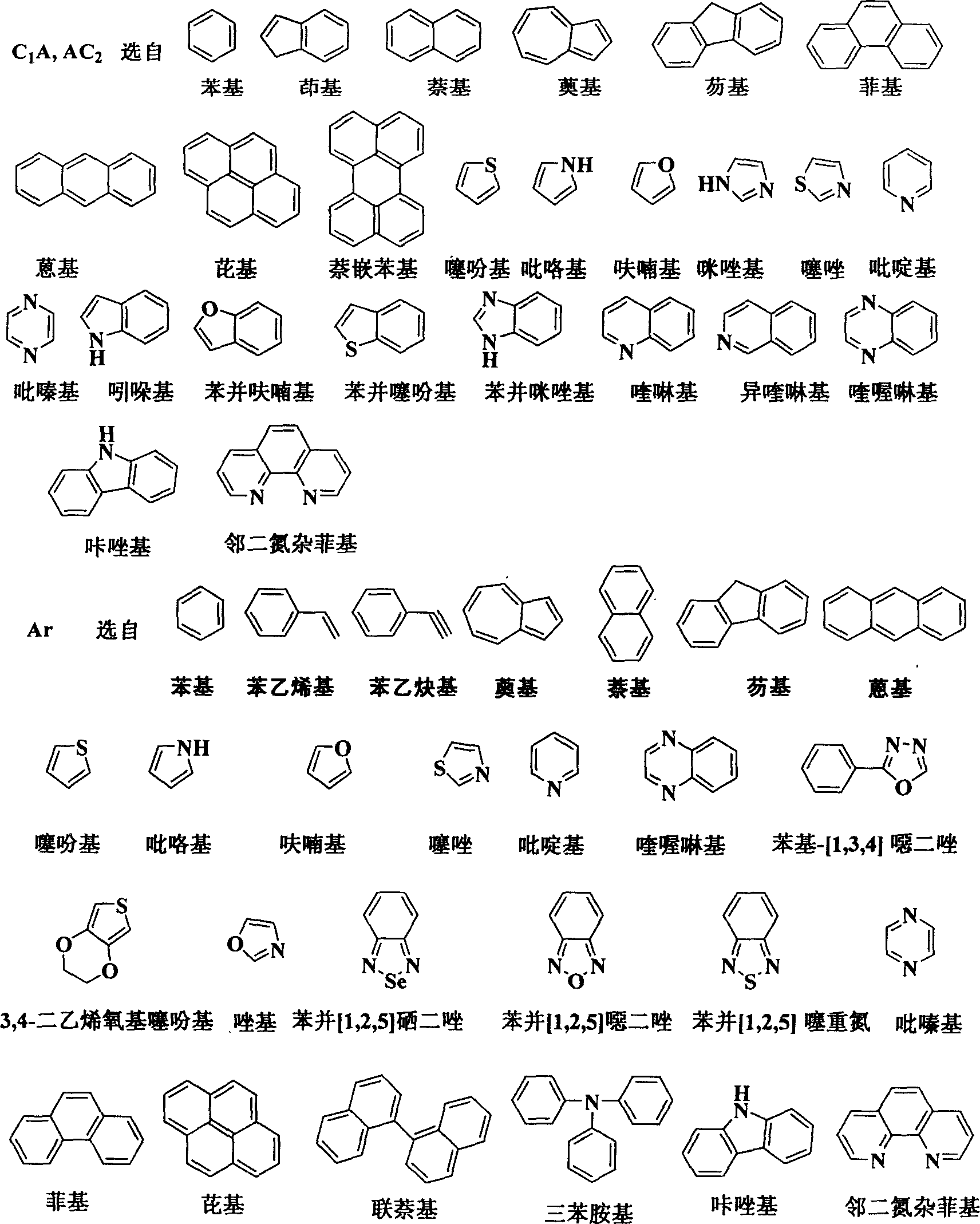
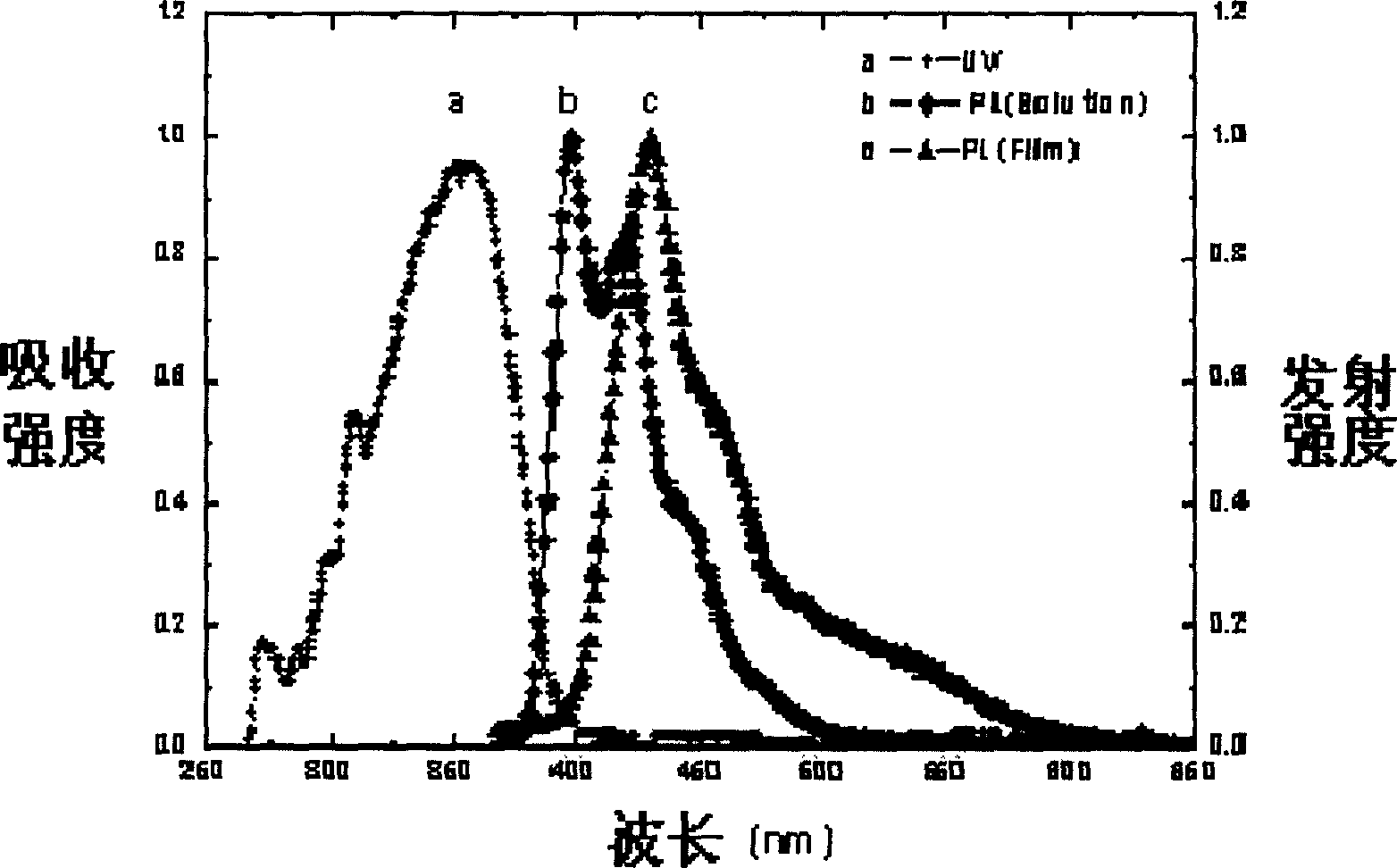
Spirofluorene materials containing non-benzene aromatic ring and synthesis and use thereof
An aromatic ring, non-benzene technology, applied in the field of spirofluorene oligomers and polymer materials and their preparation, can solve the problems of spirofluorene system without articles and patent reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
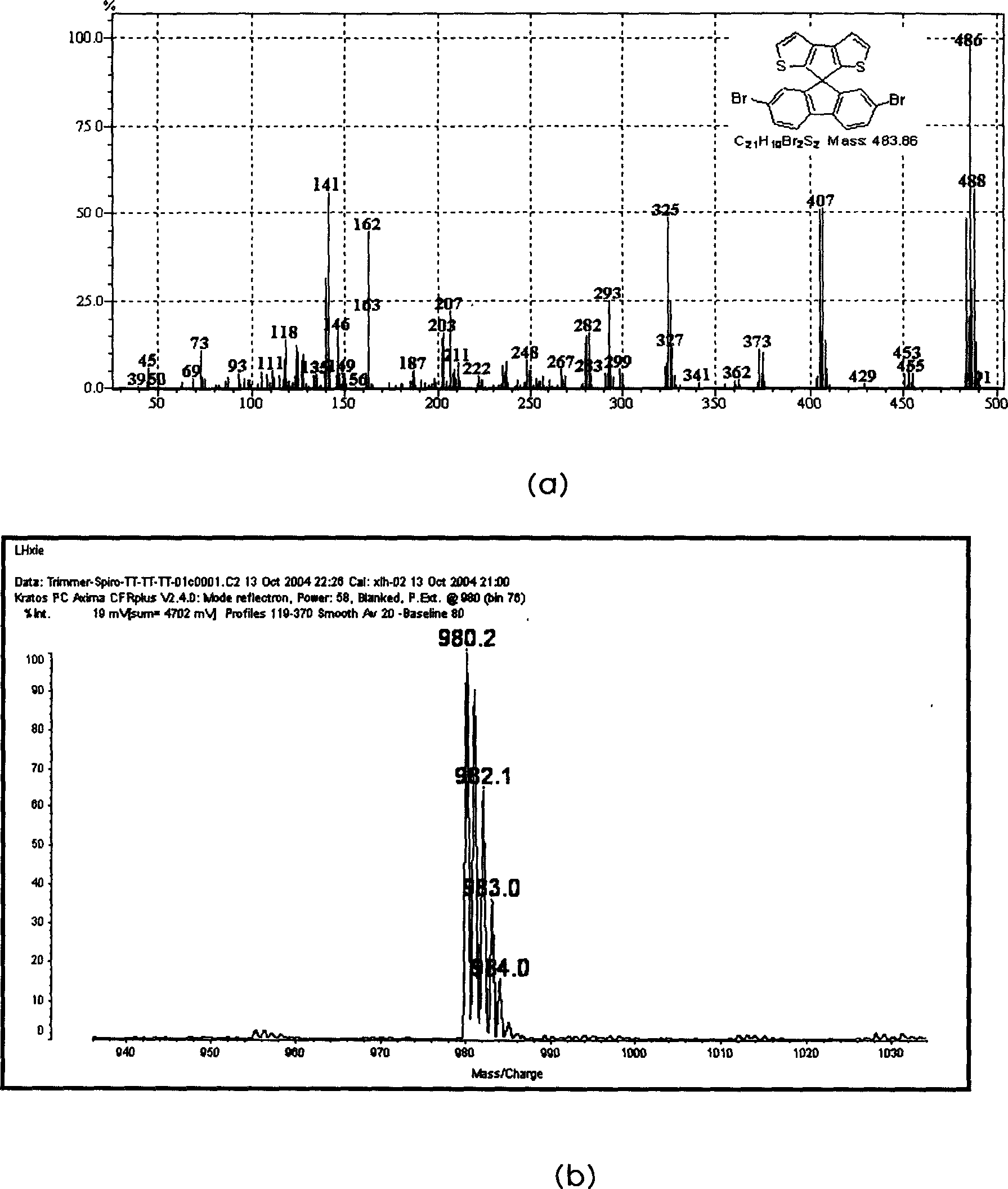
[0046] Example 1. Non-Suzuki coupling reaction Containing thiophene non-benzene aromatic ring spirofluorene unit and its oligomer trimer compound: spiro[pentacyclo[1,2-b:4,3-b']dithiophene-4 , 9'-2', 7'-(dispirobifluorene-2,2-yl)fluorene]
[0047]
[0048] 3,3-dithiophene
[0049] Add n-butyllithium (n-BuLi, 66.72mL, 106.75mmol, 1.6mol / L in Hexane, 1 equiv.) dropwise to 250mL (0.424mol / L) of -78°C tetrahydrofuran produced from acetone and dry ice; then , dropwise added 10 mL of tribromothiophene (106.75 mmol, 1 equiv.) to react for 45 minutes, then added 15.735 g (1.1 equiv.) of copper chloride at -78°C to react for one hour, then returned to room temperature and reacted for another 2 hours. The reaction was quenched with water, extracted with ether, dried and rotary evaporated, and purified by petroleum ether silica gel column to obtain 4.51 g of white crystal 3,3-dithiophene (51% yield). MS (M + =166).
[0050] 2-bromo-3,3-dithiophene
[0051] Take 2.075g (12.5mmol) ...
Embodiment 2
[0059] Example 2, Suzuki polycondensation reaction to prepare trimer oligomers containing thiophene non-benzene aromatic ring spirofluorene units: trimeric spiro[pentacyclo[1,2-b:4,3-b']dithiophene-4, 9′-fluorene]
[0060]
[0061] Take 2'-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-spiro[pentanecyclo[1,2-b:4,3-b' ]dithiophene-4,9'-fluorene] 1.23g (1 equiv., 2.71mmol) and 2',7'-dibromospiro[pentanecyclo[1,2-b:4,3-b']dithiophene -4,9'-fluorene] 0.6597g (2.0equiv., 1.36mmol) mixed and dissolved in 20ml of toluene and tetrahydrofuran mixed solvent, add catalyst Pd (PPh 3 ) 4 (156.6mg, 5mol%), avoiding light and passing nitrogen, then adding K 2 CO 3 1.71ml 2.71ml (2mol / L, 2equiv.) reacted at 90°C for 48 hours, added water after the reaction, and used CHCl 3 Extraction, drying and rotary evaporation, petroleum ether: dichloromethane mixed solvent (5:2) silica gel column purification, to obtain white solid trimeric spiro[pentanecyclo[1,2-b:4,3-b']dithiophene-4 , 9'-fluor...
Embodiment 3
[0062] Example 3, Suzuki reaction to prepare alternating copolymers: poly(9,9-di-n-octane fluorene)-copolymerization-(9,9'-spiro[pentane[1,2-b:4,3-b'] Dithiophene-4,9'-fluorene])
[0063]
[0064] 9,9-Di-octanefluorene-2,7-bis(trimethylene borate)
[0065] Take 2,7-dibromo-9,9-n-octane fluorene (1 equiv.) and magnesium chips (2.1quiv.) in THF to react, then slowly add the Grignard reagent generated by it to the mixture made of dry ice and Acetone produced -78 ° C in excess triisopropyl borate (3.0 equiv.). After the mixture was reacted at -78°C for 2 hours, the mixture was hydrolyzed in 5% glacial sulfuric acid. Then it was extracted with ether, rotary evaporated, and finally recrystallized in n-hexane / acetone. The obtained bisboronic acid continues to react with 1,3-propanediol to generate 9,9-di-n-octane fluorene-2,7-bis(trimethylene borate) 1 H NMR (200MHz, CDCl3) δ7.78(d, J=7.7Hz, 2H), 7.72(s, 2H), 7.71(d, J=7.4Hz, 2H), 4.21(t, J=5.4Hz, 8H ), 2.12-1.94(m, 8H), 1.32...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal decomposition temperature | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com