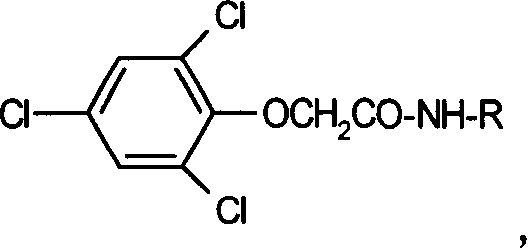
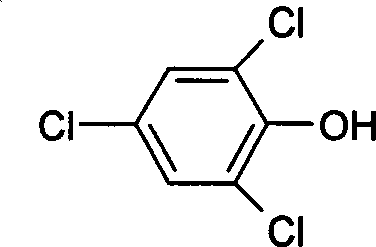
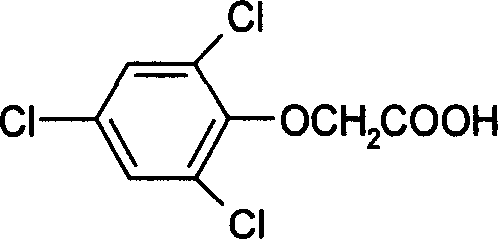
2, 4, 6-trichlorophen artificial antigen, and its preparing method and use
A technology of artificial antigen and trichlorophenol, which is applied in the field of fluorescence immunoassay and achieves the effects of strong practicability, good antibody stability, and simple and feasible preparation technology.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1 Synthesis and identification of 2,4,6-trichlorophenol hapten
[0050] 1 Synthesis of 2,4,6-trichlorophenol hapten
[0051] Dissolve 4.5g (47.6mmol) of chloroacetic acid in 7.5ml of water, slowly add 2.55g of sodium carbonate powder under stirring, and the pH of the solution is 7.5. Take 6.7g (38.1mmol) of 2,4,6-trichlorophenol, 10ml of water, and 5ml of 25% sodium hydroxide, and dissolve them completely. The pH of the solution is 8, and then add the above-mentioned sodium chloroacetate solution under stirring, and keep the pH at 7~ 8 (adjusted with dilute alkali), add 0.08g of catalyst polyethylene glycol-6000, continue to heat the reaction for 2h, while paying attention to controlling the pH of the reaction solution to 9~10 (adjusted with dilute alkali), keep warm at 80~90°C for 3h, the reaction is completed, Add a small amount of activated carbon for decolorization, boil for 10 minutes, filter while hot, adjust the pH of the filtrate to 1 with hyd...
Embodiment 2
[0054] Example 2 Synthesis and Identification of 2,4,6-Trichlorophenol Artificial Antigen
[0055] The hapten 2, 4, 6-trichlorophenol phenoxyacetic acid is connected to BSA and OVA by the activated ester method and the mixed anhydride method respectively, to synthesize artificial antigens (immunogen and coating former). The specific methods are:
[0056] 2.1 Synthesis of immunogen
[0057] Weigh 0.2mmol 51mg hapten 2,4,6-trichlorophenol phenoxyacetic acid and dissolve in 200μl DMF, dissolve 19.4mg NHS and 34.2mg DCC in 300μl DMF, add dropwise to the hapten solution, stir at room temperature React for 8 hours, overnight at 4°C, and centrifuge to separate the supernatant. The activated ester solution in the upper layer was added to 60mg BSA dissolved in 5ml carbonate buffer solution (0.05M, pH9.6), and reacted at 4°C for 4h. After the reaction was completed, the solution was put into a dialysis bag, dialyzed with distilled water for 2 days, and dialyzed with pH7.0 ph...
Embodiment 3
[0064] Example 3 Preparation and purification of 2,4,6-trichlorophenol antibody
[0065] 3.1 Preparation of antiserum
[0066] Select two adult male New Zealand white rabbits from each group (number 1 and II), weighing about 2-2.5Kg, and raise them in a standard experimental animal room. After one week of observation, they will be immunized (raised by the animal room of the Agricultural College of Shanghai Jiao Tong University).
[0067] Slowly thaw the immunogen, dilute it to 1 mg / ml with 0.9% normal saline (or phosphate buffer solution), inhale it into a 5ml sterile syringe according to the immune dose, add an equal amount of complete Freund's adjuvant (CFA) for the first immunization, and use poly The vinyl fluoride plastic tube is connected to another 5ml syringe, and pushed until it is fully emulsified to form a water-in-oil (W / O) state, and the immunization is injected according to the immunization plan.
[0068] For the first immunization, the immunogen emulsif...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com