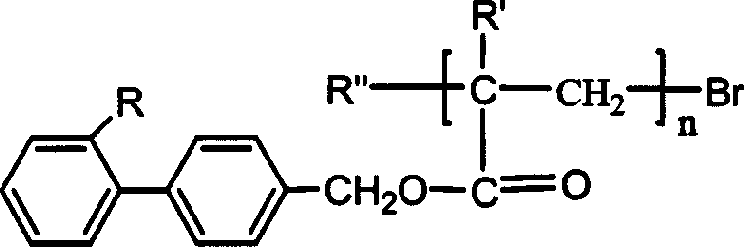
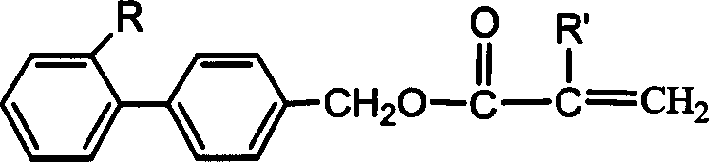
Acrylic ester and methyl acrylic ester kind side chain type luminous polymer and its synthesis
A technology of methacrylic acid and light-emitting polymer, applied in the directions of light-emitting materials, chemical instruments and methods, etc., can solve the problems of short service life of devices, low light-emitting efficiency of devices, urgent improvement of device stability, etc., and achieves a wide reaction temperature range. , the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Synthesis of 4-(2'-cyanobiphenyl)methyl (meth)acrylate
[0037] 1. Hydrolysis of 2-cyano-4'-bromomethylbiphenyl
[0038] In 800ml of dilute alkali (potassium hydroxide or sodium hydroxide) with a concentration of 0.3 to 1%, add 1 to 3 g of 2-cyano-4'-bromomethylbiphenyl and heat to reflux, and filter off unreacted raw materials while hot. After cooling, crystals were precipitated, filtered and dried to obtain the hydrolyzed product 2-cyano-4'-hydroxymethyl biphenyl.
[0039] 2. Synthesis of 4-(2'-cyanobiphenyl)methyl (meth)acrylate
[0040] Take 4.0g of the above-mentioned hydrolyzed product 2-cyano-4'-hydroxymethyl biphenyl and dissolve it in 60-100ml tetrahydrofuran, add 7.0-9.0ml triethylamine under ice bath, and take another 4.0-6.0ml (methyl) Acryloyl chloride is dissolved in tetrahydrofuran (the volume ratio of the two is 1:4), and then added dropwise to the above solution, reacted under ice bath for 2-3 hours, and then reacted at room temperature for...
Embodiment 2
[0041] Example 2: Synthesis of (meth)acrylic acid-4'-(2-carboxybiphenyl)methyl ester
[0042] 1. Hydrolysis of 4'-bromomethylbiphenyl-2-carboxylate
[0043] In 800ml of 0.3-1% dilute alkali (potassium hydroxide or sodium hydroxide), add 2-4 grams of 4'-bromomethylbiphenyl-2-methyl carboxylate and heat to reflux, and filter off unreacted raw materials while hot , cooled, crystals were precipitated, filtered and dried to obtain the hydrolyzed product 4'-hydroxymethylbiphenyl-2-carboxylic acid.
[0044] With other 4'-bromomethylbiphenyl-2-carboxylic acid alkyl esters as raw materials, corresponding reactions can also occur to obtain 4'-hydroxymethylbiphenyl-2-carboxylic acid.
[0045] 2. Synthesis of (meth)acrylic acid-4'-(2-carboxybiphenyl)methyl ester
[0046]Take 4.2g of the above hydrolyzed product 4'-hydroxymethylbiphenyl-2-carboxylic acid and dissolve it in 60~100ml tetrahydrofuran, add 7.0~9.0ml triethylamine under ice bath, and take another 4.0~6.0ml (meth)propylene Th...
Embodiment 3
[0047] Embodiment Three: Synthesis of (meth)acrylate-4-(biphenyl)methyl ester
[0048] 1. Hydrolysis of 4-bromomethylbiphenyl
[0049] In 800ml of 0.3-1% dilute alkali (potassium hydroxide or sodium hydroxide), add 1-3g of 4-bromomethylbiphenyl and heat to reflux, filter off unreacted raw materials while hot, cool down, precipitate crystals, filter and dry In the hydrolysis product 4-hydroxymethyl biphenyl.
[0050] 2. Synthesis of 4-(biphenyl)methyl (meth)acrylate
[0051] Take 3.6g of the above-mentioned hydrolyzed product 4-hydroxymethylbiphenyl and dissolve it in 60-100ml tetrahydrofuran, add 7.0-9.0ml triethylamine under ice bath, and take another 4.0-6.0ml (meth)acryloyl chloride and dissolve it in tetrahydrofuran ( The volume ratio of the two is 1:4), and then added dropwise to the above solution, reacted under ice bath for 2-3 hours, and then reacted at room temperature for 8-16 hours. The reactant was washed successively with dilute alkali and dilute acid to obtain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com