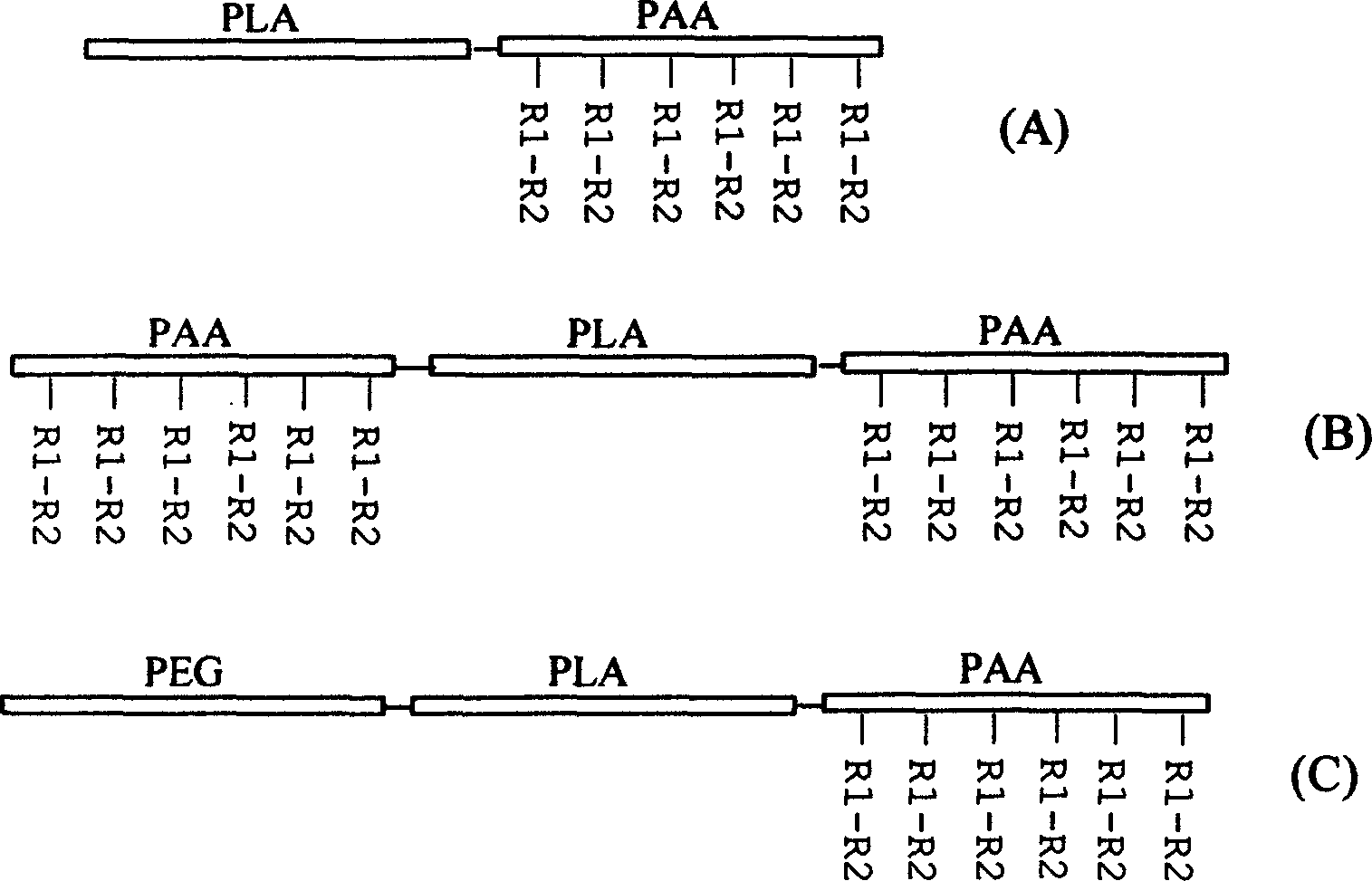
Aliphatic polyester-polyamino acids copolymer with biological function and its synthesis method
A technology of aliphatic polyester and polyamino acid, applied in the field of high molecular polymer biomedical materials, can solve problems such as difficult modification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Preparation of polyethylene glycol-polylactide-polyglutamic acid block copolymer by hydrogenation reduction.
[0030] (1) 0.5g polyethylene glycol-aliphatic polyester-polybenzyl glutamate is dissolved in 15ml of tetrahydrofuran, add 5ml of methyl alcohol and a small amount of acetic acid, then add 0.1g of palladium / carbon (10%) as Catalyst, stirred and reacted for a certain period of time under hydrogen bubbling at room temperature, filtered to remove palladium / charcoal, settled with ether, filtered, and vacuum-dried to obtain the product. The results are shown in Table 1.
[0031] make up
[0032] (2) In the autoclave, add 1g of polyethylene glycol-polylactide-polybenzyl glutamate (DP PEG =16,DP PLA =44,DP PBLG =63), dissolve with 30ml dioxane and 10ml methanol, then add a small amount of acetic acid and 0.2g palladium / carbon (10%) as catalyst, react for a certain period of time at 50 ℃ 2 and 8atm, remove palladium / carbon by filtration, use Diet...
Embodiment 2
[0035] Example 2: Preparation of polyethylene glycol-polycaprolactone-polyglutamic acid block copolymer by hydrogenation reduction.
[0036] A certain amount of polyethylene glycol-polycaprolactone-polybenzyl glutamate was added to the reactor, and other reaction steps were the same as in Example 1 to obtain polyethylene glycol-polycaprolactone-polyglutamate segment copolymers. The yield is above 90%.
[0037] Numbering
Embodiment 3
[0038] Example 3: Preparation of polyethylene glycol-polyglycolide-polyglutamic acid block copolymer by hydrogenation reduction.
[0039] A certain amount of polyethylene glycol-polyglycolide-polyglutamic acid benzyl ester is added in the reactor, and other reaction steps are the same as in Example 1 to obtain polyethylene glycol-polyglycolide-polyglutamic acid (Molecular weight PEG=2000, PGA=1000, PLG=6200) block copolymer. The yield is about 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com